Heart failure (HF) is associated with significant morbidity and mortality, and the burden of disease is rising.1 Despite improved survival – partly a result of advances in medical therapy, coronary interventions and ICD – the mortality rate remains high and relatively stagnant.2 Moreover, advanced HF is associated with impaired quality of life (QOL), which is reflected in the significant number of hospitalisations and increased healthcare costs.
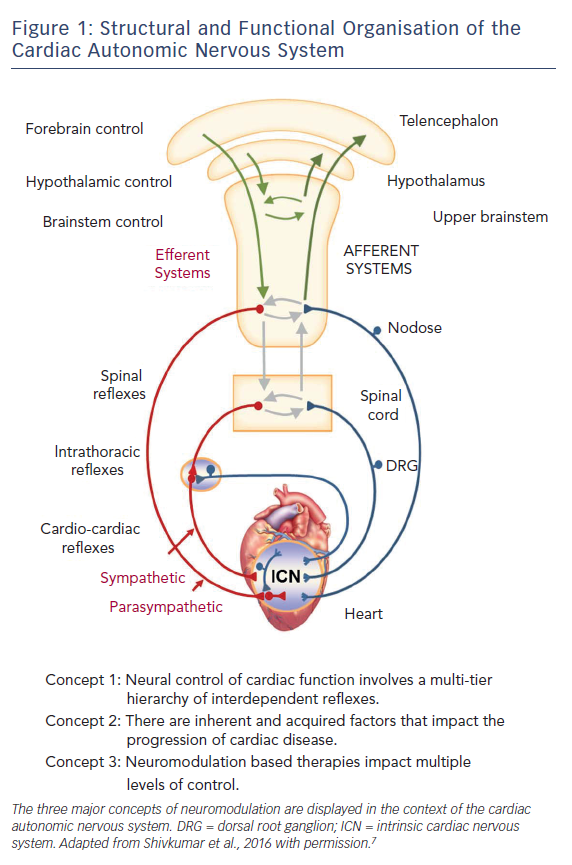
The aetiologies of HF are varied but autonomic dysfunction is a hallmark. Imbalance in the complex and dynamic interactions between the sympathetic and parasympathetic efferent limbs of the autonomic nervous system (ANS) is not only reactive to HF as a means of maintaining homeostasis, but also a contributor to HF progression. The interplay between multiple levels of the hierarchy for cardiac control (Figure 1) ultimately results in excessive sympathetic responses with corresponding withdrawal of parasympathetic tone. Furthermore, depressed arterial baroreflex regulation, a major contributor to reflex control of cardiac and peripheral vascular function, is associated with poor survival.3–5 For additional details regarding the pathophysiology of the ANS in HF, we refer the reader to recent articles on the subject.6–8
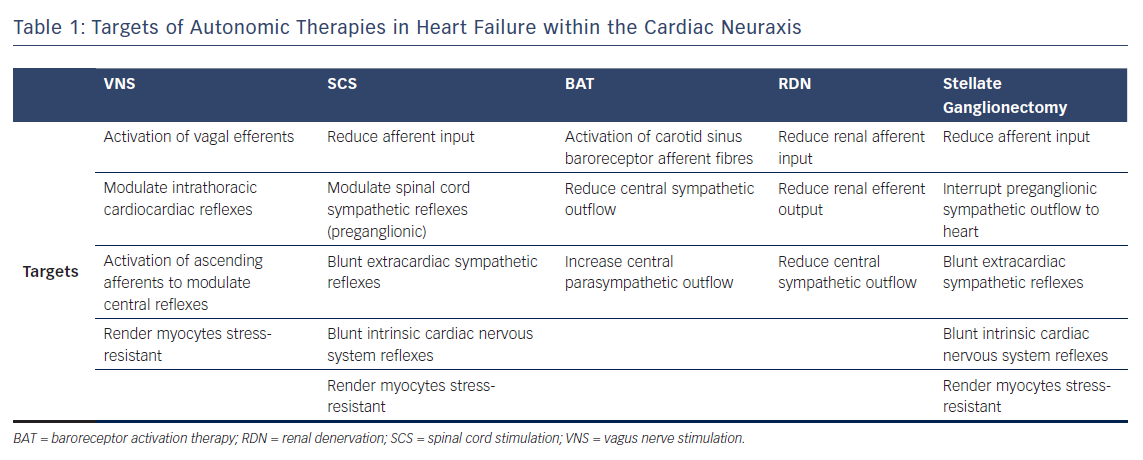
Medical approaches to treating autonomic dysfunction in HF focus on reducing the overactive sympathetic nervous system through the blockade of the beta-adrenergic or renin–angiotensin–aldosterone systems. However, despite improvements in pharmacologic approaches, treatment of HF remains challenging.9,10 Neuromodulation therapy to restore sympathovagal balance in HF has garnered increasing interest in recent years. Emerging therapies in this area include vagus nerve stimulation (VNS), spinal cord stimulation (SCS), baroreflex activation therapy (BAT), renal denervation (RDN) and stellate ganglionectomy (Figure 2). Here, we summarise the current data in animal models and clinical studies on these autonomic therapies in HF as well as challenges to the implementation of these treatment modalities.
Approach to Cardiac Neuromodulation
When considering the application of bioelectric therapies for cardiac disease, three main concepts of neurocardiology merit discussion.11 Firstly, neural control of cardiac function is exerted through the interactions between central and peripheral components of the cardiac ANS (Figure 1).8 Secondly, the aforementioned interactions may be weakened or strengthened depending on the level of the cardiac neuraxis and the characteristics of the underlying cardiac pathology.12–14 Such neural remodelling is critically dependent on abnormal afferent input.7,8,15,16 Lastly, as the neuromodulation acts on axons of passage, associated neural networks (above and below site of intervention) and the heart itself, the outcome of neuromodulation depends on the stimulation parameters, the location within the neuraxis in which therapy is applied and the cardioneural pathologic substrate against which the therapy is applied. It is highly likely that the optimum neuromodulation approach may be different depending on the status of the patient and that even within a given patient, therapy will need to be adjusted with time, as is already done for pharmacologic approaches.
Vagus Nerve Stimulation
VNS devices were initially developed and approved for use in the treatment of epilepsy and refractory depression.17–22 Interest in VNS has expanded to treatments for visceral disorders and for cardiac pathologies.8,22,23 The central premise of VNS is to increase parasympathetic tone and to restore reflexes that mitigate adrenergic inputs to the heart (Table 1). Additionally, VNS is cardioprotective because it limits cardiomyocyte apoptosis and inflammation.24–26 It also protects the heart by altering substrate use within the heart muscle itself.27,28 At the molecular level, VNS may improve survival through the reduction in connexin 43 loss and promotion of electrical stability.29
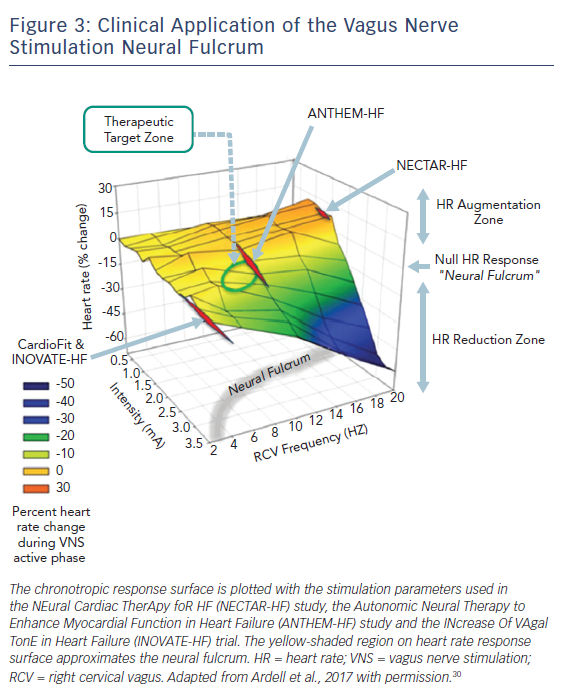
When delivered to the cervical vagosympathetic trunk, VNS activates both ascending (afferent) and descending (parasympathetic efferent) projections (Figure 2). The cardiac nervous system works in a push push-back; fashion. Functional cardiac responses to afferent activation are engaged at lower stimulus intensities leading to withdrawal of centrally derived parasympathetic tone with the potential to modify sympathetic activity (Figure 3). As stimulus intensity is increased, parasympathetic efferents are engaged with expected decreases in regional cardiac function (Figure 3); excessive parasympathetic stimulation can lead to rebound effects during the off-phase of intermittent VNS.30–32 When ascending and descending projections within the cervical vagus are activated in a ‘balanced’ fashion, multiple levels of the cardiac neuraxis are engaged with little or no change in basal cardiac function – we refer to this as the neural fulcrum.30,33,34 The major effects of VNS delivered at this operating point are placing restraints on aberrant reflex processing within the peripheral neural networks of the intrinsic cardiac nervous system, rendering myocytes stress-resistant and exerting anti-adrenergic effects on the heart itself.8
Animal studies have demonstrated the efficacy of chronically implantable VNS device therapy in sudden cardiac death and HF. In an acute ischaemia model in dogs with healed MI, chronic right cervical VNS protected against VF.35 Chronic VNS at the right cervical vagus nerve stymied the progression of HF in a canine high-rate pacing model and dramatically improved LVF and survival in a rat model of HF.26,36 Chronic VNS, both left and right, was likewise effective in maintaining cardiac function in guinea pig models of chronic MI and pressure overload.27,28
The Autonomic Neural Therapy to Enhance Myocardial Function in Heart Failure (ANTHEM-HF) study evaluated the use of a VNS system (Demipulse® Model 103 pulse generator and Perennia FLEX® Model 304 lead; Liva Nova, Houston, TX, USA) in patients with HF.37 Its stimulation protocol used titration to the neural fulcrum (as defined above and depicted in Figure 3). Sixty patients with New York Heart Association (NYHA) functional class II–III symptoms, left ventricular ejection fraction (LVEF) ≤40 % and LV end-diastolic diameter (LVEDD) ≥50 mm to <80 mm underwent randomisation for implantation at either the left (n=31) or right (n=29) cervical vagus nerve. Regarding the primary safety endpoint of incidence of procedure- and device-related adverse events, one patient died 3 days after an embolic stroke that occurred during implantation. An additional 20 serious adverse events occurred, but none of these were attributed to the VNS system or its implantation. There were statistically significant improvements in the primary efficacy endpoints of LVEF and LV end-systolic volume (LVESV) as well as the secondary efficacy endpoints of LV end-systolic diameter (LVESD), heart rate variability and 6-minute walk test (6MWT). Although there was a trend for improved efficacy outcomes with right as opposed to left VNS, CIs were wide, and there were no statistically significant differences in most efficacy parameters or safety profiles. Subsequent 12-month follow-up on 49 of the initial 60 patients showed that improvements persisted during longer follow-up and that the device implantation remained safe.38 While this study focused on HF with reduced ejection fraction (HFrEF), the ANTHEM-HF with preserved ejection fraction (HFpEF) study seeks to evaluate the safety and efficacy of right cervical VNS in patients with HFpEF and HF mid-range ejection fraction using a similar stimulation protocol and with at least 12-month follow-up.39 Results should be available in late autumn 2018.
In contrast to the results from ANTHEM-HF, two other recent studies using VNS produced more neutral effects, at least with respect to objective outcomes such as echocardiographic parameters. The NEural Cardiac TherApy foR HF (NECTAR-HF) study was a randomised, sham-controlled trial that evaluated the utility of VNS using the Precision Spectra™ system (Boston Scientific; St Paul, MN, USA).40 In this study, 96 patients with NYHA functional class II–III symptoms, LVEF ≤35 % and LVEDD ≥55 mm were randomised to VNS or control (device implanted but VNS off) in a 2:1 ratio for a 6-month period. Stimulation intensity was titrated, as tolerated, with a target of 20 Hz, a duty cycle of 16.7 %, a pulse width of 300 µs and a proposed maximal current intensity of 4 mA. However, primarily because of off-target effects, stimulus intensity was ~1.4 mA, which is in the region below the neural fulcrum and sub-threshold for optimal stimulation (Figure 3).40 In an analysis of 87 of the 96 patients implanted with available data, there was no statistically significant change in the primary endpoint of LVESD. Regarding adverse events, one patient died in the postoperative period from a pulmonary embolism, and there were three patient deaths between randomisation and 6 months as a result of worsening HF or HF complications. Of the 96 patients in the initial 6-month study, 91 patients were evaluated for a total of 18 months. All devices were activated after the initial 6-month period. Those in the group that crossed over from the control group to VNS activation had decreases in LVESV without significant changes in LVESD and LVEF.41
The INcrease Of VAgal TonE in Heart Failure (INOVATE-HF) trial evaluated the CardioFit system (BioControl Medical, Yehud, Israel) in advanced HF.42 This system used a combination of R-wave triggered VNS pulse delivery with a putative afferent blockade component. In this study, 707 chronic HF patients with NYHA functional class III symptoms and LVEF ≤40 % were randomised to VNS or continued medical therapy in a 3:2 ratio and were followed for a mean of 16 months. Four weeks after implantation, patients in the VNS group underwent stimulation intensity adjustment with a target of 3.5–5.5 mA. Figure 3 illustrates the position of this stimulation protocol in relation to the overall VNS response surface. While the secondary endpoint outcomes of NYHA functional class, QOL and 6MWT improved in the VNS group, the primary efficacy endpoint – a composite of death or HF hospitalisation and/or IV diuretic use – occurred more often in the VNS group than in the control group. The trial achieved the co-primary safety endpoint with a rate of freedom from procedure- and system-related events of 90.6 %. The study was negative in that VNS did not reduce the rate of death or HF events in chronic HF patients.
Spinal Cord Stimulation
SCS is a Food and Drug Administration-approved therapy for chronic pain syndrome and refractory angina. High thoracic SCS has been used for the treatment of angina caused by coronary artery disease since the 1980s.43–47 Rather than being solely restricted to the spinal cord, SCS is now thought to act at multiple points within the cardiac neuraxis (Table 1).8,48 SCS suppresses the release of cardiac-related afferent neurotransmitters within the dorsal horn of the spinal cord, modulates sympathetic preganglionic neural activity, reduces sympatho-excitation within the intrathoracic extracardiac ganglia and blunts the intrinsic cardiac nervous system reflex response to cardiac stressors (Figure 2).49 SCS has additional cardioprotective effects including reducing arrhythmia burden and apoptosis, while improving contractile function.50–53 In a rabbit model of transient acute ischaemia, SCS reduces infarct size through inhibition of cardiac adrenergic neurons.54 In canine models of healed MI and pacing-induced HF, SCS improved contractile function and reduced the risk of ventricular arrhythmias, plasma brain natriuretic peptide (BNP) and norepinephrine levels.53,55 SCS has also been shown to improve contractile function and myocardial oxygen consumption in a porcine model of MI-induced HF.56
Two clinical studies have evaluated the efficacy of SCS in HF. Thoracic Spinal Cord Stimulation for Heart Failure as a Restorative Treatment (SCS HEART) was a non-randomised, open-label pilot study of 22 patients with NYHA functional class III symptoms and LVEF 20–35 % with ICD on stable, optimal medical therapy with LVEDD of 55–80 mm.57 Seventeen patients underwent implantation of the Eon Mini™ Neurostimulation System (St Jude Medical; Plano, Texas, USA) at levels T1–3 with SCS parameters of 24 h/day, frequency of 50 Hz and pulse width of 200 µs. The primary efficacy endpoint was a composite of six parameters, of which there was significant improvement in NYHA class, QOL, peak maximum oxygen consumption, LVEF and LVESV but not in N-terminal prohormone- (NT pro-) BNP. In terms of safety, there were no deaths or device-device interactions at 6 months. The Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Systolic Heart Failure (DEFEAT-HF) study was a prospective, multi-centre randomised, parallel, single-blind, controlled trial that included 81 patients with NYHA functional class III symptoms, LVEF ≤35 %, QRS duration <120 ms and LVEDD ≥55 mm.58 An eight-electrode lead Medtronic Model 3777/3877 (Medtronic; Minneapolis, MN, USA) was implanted in the epidural space with stimulation applied to levels T2–T4 at 50 Hz for 12 h/day. The primary objective of the study was to evaluate the LVESV index (LVESVi). At 6-month follow-up, there was no significant difference in LVESVi. As SCS exhibits a memory function of approximately 45 minutes for maintained efficacy in the off-phase, future studies should restrict time of the off-phase to less than 1 hour to maximise the potential for effective control of the cardiac nervous system.59
Baroreflex Activation Therapy
As the baroreceptor reflex is involved in blood pressure regulation, BAT was developed as a potential treatment for resistant hypertension.60–63 Its utility has also been demonstrated in angina.64,65 Baroreceptors are stretch receptors located in the carotid sinus and aortic arch whose soma are contained within the petrosal and nodose ganglia, respectively. Baroreceptors transmit information regarding arterial pressure centrally (Figure 2). As part of a negative feedback reflex control mechanism, sympathetic and parasympathetic outflows are thereby modulated (Table 1). In HF, baroreceptor sensitivity is reduced with increased sympathetic activity, which may be mediated, in part, by elevated angiontensin II levels.16 Through electrical stimulation of the baroreceptor afferent fibres, central sympathetic outflow is reduced while parasympathetic tone is augmented.8,66 In that regard, in the Device-based Therapy in Hypertension Trial (DEBuT-HT), 45 patients with refractory hypertension undergoing implantation of a carotid stimulator Rheos® System (CVRx; Minneapolis, MN, USA) had significant blood pressure drop at 2-year follow-up.67
Preclinical studies in HF have produced proof-of-concept for BAT efficacy. In a canine model of MI-induced HF, BAT was shown to increase LVEF, reduce LVESV, LV end-diastolic pressure and circulating plasma norepinephrine, as well as normalising expression of cardiac beta1-receptors, beta-adrenergic receptor kinase and nitric oxide synthase.68 On histologic examination, there was reduced fibrosis and hypertrophy. BAT has also been shown to improve survival in a pacing-induced HF canine model.69
The first-in-man pilot study was a single-centre, open-label study involving 11 patients with NYHA functional class III symptoms, LVEF <40 % on optimal medical therapy and ineligible for cardiac resynchronisation therapy (CRT) who underwent BAT for 6 months.70 This study demonstrated safety with only one hospital- and procedure-related complication of anaemia requiring transfusion with no further sequelae. Patients had reductions in muscle sympathetic nerve activity and improvement in baroreflex sensitivity, LVEF, NYHA class, QOL and 6MWT. In addition, there was a decreased rate of HF hospitalisations compared with the 12-month period prior to BAT system implantation. The Barostim neo system (CVRx), which has approval in Europe, has been evaluated in the Barostim Hope for Heart Failure (HOPE4HF) trial. This randomised controlled trial included 146 patients with NYHA functional class II symptoms and LVEF ≤35 %.71 Patients who underwent BAT improved in NYHA functional class, QOL, 6MWT, and had a reduction in NT pro-BNP. There was a trend towards reduction in HF hospitalisations. However, there were no changes in echocardiographic parameters, including LVEF. Given the evidence that CRT reduces the sympathovagal imbalance in HF, a subsequent analysis demonstrated that the most pronounced effect of BAT was in patients not treated with CRT.72,73 This study will be followed by the Baroreflex Activation Therapy® for Heart Failure BeAT-HF) trial, which seeks to randomise 480 patients with NYHA functional class III symptoms and LVEF ≤35 %. Primary outcomes will be cardiovascular and HF mortality and the safety endpoint will be major adverse neurological and cardiovascular events at 6 months.
Renal Denervation
RDN was initially evaluated in the context of refractory hypertension.74,75 Its efficacy is predicated on interrupting axons (afferent and efferent) projecting along renal arteries (Table 1). The electrode catheter is positioned just proximal to the origin of the second-order renal artery branch with four to eight lesions administered circumferentially along the length of each of the two arteries (Figure 2).
Surgical RDN has been shown to have salutary effects in HF in rat and canine models.76–79 Following initially promising results in the Symplicity hypertension (Symplicity HTN-2) trial of catheter-based RDN in hypertension,74 RDN was evaluated in HF. The Renal Artery Denervation in Chronic Heart Failure-pilot (REACH-pilot) study demonstrated the safety of RDN in seven patients with HFrEF on optimal medical therapy and significantly improved 6MWT.80 The REACH study is an on-going prospective, double-blinded randomised study on the safety and effectiveness of RDN in 100 HFrEF patients. The Symplicity HF trial was a feasibility study that evaluated 39 patients with NYHA functional class II-III symptoms and LVEF <40 % on optimal medical therapy with mildly impaired renal function. In the study, one patient did experience renal artery occlusion that may have been related to the RDN procedure.81 The study showed significant reductions in NT pro-BNP without significant changes in LVEF, 6MWT, or estimated glomerular filtration rate. A major complication identified within the Symplicity trials was that substantial variability in efficacy was related to inadequate focus on the extent and verification of axon ablation from the renal artery. Future studies should be designed to assess efficacy at onset and during the course of therapy. A more recent pilot study randomised 60 HF patients with LVEF ≤40 % and NYHA functional class II–IV symptoms to RDN plus optimal medical therapy versus optimal medical therapy alone.82 No adverse effects were identified, and significant improvements were noted in the primary efficacy endpoint of LVEF at 6 months and in secondary endpoints of NYHA functional class, NT pro-BNP, heart rate and Short Form 36 health survey questionnaire in the RDN group.
Stellate Ganglionectomy
Cardiac sympathetic denervation (CSD) via stellate ganglionectomy in cardiovascular disease was initially proposed as a treatment for angina in 1899 and has since demonstrated efficacy in reducing angina and ventricular arrhythmias.83–86 The procedure involves the removal of the lower half of the stellate ganglia through the T2–T4 thoracic ganglia as a means of disrupting afferent and sympathetic postganglionic efferent fibres to the heart. Left CSD (LCSD) has been most commonly performed, although recent data suggest improved effectiveness in ventricular arrhythmia with bilateral approaches.84 A prospective, randomised pilot study has evaluated LCSD for HF.87 In this study, Conceição‐Souza et al. randomised 15 patients with LVEF ≤40 % in sinus rhythm with resting heart rate >65 BPM and on optimal medical therapy to continued medical therapy with left stellate ganglionectomy versus medical therapy alone. The study showed no complications attributed to the surgery and mild improvement in LVEF, 6MWT and Minnesota Living with Heart Failure Questionnaire. A large randomised study evaluating LCSD in systolic HF is currently enrolling.88
Challenges to Autonomic Therapies in Heart Failure
Several challenges to neuromodulation in HF help explain why success in preclinical studies has not translated into clinical benefit in human studies. In particular, just as target dosing of oral medications is critical in conventional therapy for HF, so too are the bioelectric stimulation parameters and protocols. Many trials employed distinct stimulation parameters with respect to frequency, current pulse width and duty cycle, often with minimal mechanistic justification. Cardiac disease is a dynamic process; neuromodulation is too. As a patient’s sympathovagal balance shifts during the course of the disease process, changes in stimulation parameters may be warranted. It is much more than ‘set and forget’. Future studies should also consider relevant biomarkers in assessing engagement of the neural elements and the effects on end-organ function. With such biomarkers, the potential for effective closed-loop systems can become a reality.
Autonomic imbalance plays a crucial role in the pathophysiology of HF. While pharmacologic therapies affect the ANS, limited effectiveness with these approaches has led to interest in applying neuromodulation to HF treatment. VNS has been the most extensively studied modality and the clinical trials have had mixed results, although with the caveat that stimulation parameters may not have been appropriate. Clinical studies in SCS and RDN have had similarly variable results so far. BAT has shown some promise in a pilot study, and we look forward to the results from an on-going clinical trial regarding its efficacy. Neurovisceral science holds great promise in emerging therapies for myriad disease states. To move forwards, it is crucial to understand the structure and function of the ANS and the organs that it targets. It is with anticipation that we await critical aspects of this puzzle. Its various components are being revealed by programmes such as the National Institutes of Health (NIH) – Stimulating Peripheral Activity to Relieve Conditions (SPARC) portfolio, a programme committed to furthering knowledge of nerve-organ interactions and advancing development of neuromodulatory approaches for disease treatment.