More than a century after its discovery, Chagas disease still is a major health problem in Latin America, with 5.7 million people in 21 countries being affected by it.1 Moreover, about 70 million people are at risk of acquiring the illness.1 Cases of Chagas disease are now found globally; there are more than 400,000 immigrants with this disease living in Europe (mainly in Spain, Italy and France) and the United States.2 The consequence of this is that the annual global (direct plus indirect) cost of the disease is in the region of US $7.2 billion, which is more than the cost of the majority of cancers.3
Chagas disease is caused by the protozoan Trypanosoma cruzi, which is transmitted to humans through the faeces of insect vectors known as triatomine bugs. About 20 % of patients develop chronic cardiomyopathy within 20 years of initial diagnosis by positive serology test.1 Chagas cardiomyopathy can clinically manifest as precordial chest pain,4 thromboembolism,5 cardioembolic stroke,6 intraventricular conduction disturbances, atrioventricular block, ventricular dysrhythmias,7 sudden cardiac death (SCD)8 or chronic heart failure (CHF).9
Chagas heart failure has several peculiarities that distinguish it from other forms of heart failure. Physicians must therefore be alert to how to deal with Chagas heart failure to ensure patients receive the best treatment for this condition. This overview is intended to provide insights into how to recognise and treat patients living in non-endemic countries who have Chagas heart failure.
Epidemiology
Up to 14 % of patients in areas where Chagas disease is endemic have Chagas heart failure.7 This percentage is higher than the 2 % seen in a cohort of Chagas disease patients from a non-endemic area.10 Where the disease is endemic, asymptomatic left ventricular dysfunction affects 3–39 % of patients with chronic Chagas disease11 and precedes CHF;12 in non-endemic areas, the prevalence of this abnormality is 4 %.10
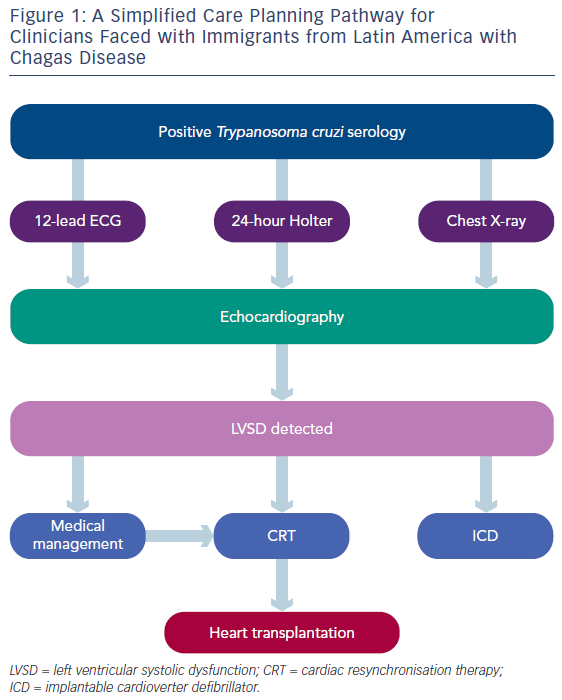
Given the epidemiology of Chagas disease, clinicians faced with immigrants from Latin America should have a high degree of suspicion for heart failure in those with positive serology. Figure 1 provides a simplified care pathway. An echocardiographic study should be undertaken to detect early myocardial dysfunction in this patient population.
The Distinctive Clinical Picture
Chagas heart failure is the consequence of reduced left ventricular ejection fraction (LVEF).9 In general, dyspnoea is the predominant symptom. Following this inaugural symptom, pedal oedema supervenes, and the clinical picture of right-sided heart failure ensues. Isolated right-sided heart failure, although rare, can be observed in patients with Chagas heart failure. It sometimes precedes the appearance of left-sided heart failure. In the presence of bi-sided heart failure, physical examination may suggest that right-sided heart failure predominates.13 This means that a patient with anasarca and left ventricular systolic dysfunction, for example, may have no dyspnoea.
In patients with mild symptoms, especially in those in New York Heart Association (NYHA) Class II, precordial chest may simulate coronary artery disease (CAD).4 Coronary angiography can be performed to rule out concomitant obstructive CAD, but this is normal in the vast majority of cases.14 Abnormalities in myocardial perfusion scintigraphy can be observed in the absence of concomitant obstructive CAD in Chagas heart failure patients, thus suggesting derangement of the coronary microvasculature.
Physical examination usually reveals a high frequency of splitting of the second heart sound, as first noted by Chagas.15 This is associated with the presence of right bundle branch block (RBBB) on 12-lead electrocardiogram (ECG). Isolated liver enlargement heralding the appearance of right-sided heart failure can also be detected on physical examination.16
Another finding not observed at the same magnitude in non-Chagas disease heart failure is the presence of cardiac arrhythmias on physical examination.17 Atrial fibrillation is observed in up to 30 % of patients with this condition18,19 and premature contractions (ventricular in origin in the vast majority of cases) occur in about 50 % of patients.18 It is important to emphasise that the prevalence of atrial fibrillation is higher in patients with Chagas heart failure than in those from a general, unselected population;20 about three-quarters of patients with Chagas heart failure will have cardiac arrhythmia on physical examination.
Peculiarities of Subsidiary Tests
RBBB and left anterior fascicular block are found in about 40 % of patients with Chagas heart failure, which is higher than that seen in CHF due to other types of cardiomyopathy.17,18,21 The association between RBBB and left anterior fascicular block, which is more frequent in patients with Chagas cardiomyopathy,22 should alert physicians to the possibility of Chagas heart failure. In contrast, the prevalence of left bundle branch block (LBBB), which is frequently found in patients with non-Chagas cardiomyopathy,23 is lower in Chagas heart failure.17,18,21 Such electrocardiographic peculiarities should raise suspicion of Chagas disease aetiology in patients with CHF coming from an endemic area.
On chest X-ray it is common to see an impressive cardiomegaly with no evidence of congestion, a finding observed as far back as 1945.13 This is probably a consequence of the concomitant right ventricular involvement usually observed in patients with Chagas heart failure. Such a characteristic should prompt physicians to consider this diagnosis in patients from an area where Chagas is endemic.
On echocardiographic study, about 37 % of patients with Chagas cardiomyopathy have segmental wall motion abnormalities similar to those found in patients with ischaemic cardiomyopathy.18 In such circumstances, it is mandatory to rule out the diagnosis of CADinduced cardiomyopathy. Another distinctive finding is the presence of apical left ventricular aneurysm in the absence of CAD, which affects about 21 % of patients with Chagas cardiomyopathy22 and is therefore highly suggestive of Chagas disease.24 This finding may help physicians diagnose patients who have emigrated from an endemic area and is very important because apical left ventricular aneurysm is associated with SCD in patients with this condition,25 probably because the abnormality is the site from which malignant ventricular arrhythmias may arise.8
Twenty-four-hour Holter monitoring can reveal complex premature ventricular contractions, particularly non-sustained ventricular tachycardia (NSVT). Holter monitoring reveals NSVT in up to 50 % of patients with Chagas heart failure,26 a much higher frequency than observed in patients with non-Chagas heart failure.27 NSVT is heralded by the presence of premature ventricular contractions on resting ECG.28 This is of the utmost importance because about 10 % of Chagas disease patients with NSVT progress to sustained ventricular tachycardia,29 which in about 24 % of cases degenerates into ventricular fibrillation and SCD.30 Clearly, the presence of NSVT in a young patient with CHF from an endemic area should raise the diagnostic possibility of Chagas heart failure.
Twelve-lead ECG and Holter monitoring can detect complete atrioventricular block. Chagas disease is the leading cause of complete atrioventricular block in areas where the disease is endemic;31 it was found in about 10 % of patients seen in one referral centre for the treatment of Chagas disease.32 In other referral centres specialised in heart failure treatment, however, the frequency of pacemakers in patients with Chagas heart failure is as high as 50 %.33 The presence of CHF and advanced atrioventricular block in young patients from an endemic area should therefore alert the physician to a diagnosis of Chagas cardiomyopathy.
Clinical Course
The clinical course of Chagas heart failure is relentless, with an annual mortality approaching 20 %.34 Predictors of mortality are NYHA Functional Class IV, LVEF, hyponatremia, lack of beta-blocker therapy and digoxin use.34 Life expectancy for patients with CHF secondary to Chagas cardiomyopathy is poorer than that observed in patients with mild to moderate CHF secondary to idiopathic dilated cardiomyopathy,21 ischaemic cardiomyopathy18 and hypertensive cardiomyopathy.17
Thromboembolism is a constant threat for patients with Chagas heart failure, particularly pulmonary embolism. It has a considerable impact on the clinical course of the disease. In severe CHF, right-sided cardiac thrombosis is found in about 58 % of patients, and half will experience pulmonary thromboembolism.35 Cerebrovascular accidents are also a clinical problem, affecting about 11 % of patients.36 In those with milder forms of the syndrome, the risk of death from cerebrovascular accidents is high, at about 42 %.26 Patients with Chagas heart failure are at risk of thromboembolic phenomena due to the high prevalence of mural thrombosis, therefore physicians should routinely screen such patients for intracavitary thrombus and provide timely anticoagulation.
Treatment
There is no evidence base supporting the treatment of patients with Chagas heart failure; Chagas disease is one of the most important but neglected diseases in the world. Treatment of this condition therefore mainly relies on the experience of physicians dealing with this syndrome.
Medical
Diuretics, particularly furosemide, have successfully been used to decongest systemic and/or pulmonary circulation, and no deleterious effect has been observed.9 When furosemide alone is unable to relieve symptoms at a mean daily dosage of 160 mg/day, hydrochlorothiazide can be added.9 In cases of hyponatremia, water restriction has proven useful. A combination of spironolactone plus enalapril has also shown to be of benefit in patients with Chagas heart failure.37
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers have been used in patients with Chagas heart failure without detrimental effect.37 Enalapril increased LVEF and decreased left ventricular systolic diameter on echocardiography, decreased cardiac silhouette on chest X-ray, and decreased brain natriuretic peptide serum levels in patients with this condition.37 Captopril 150 mg/day improved functional status and decreased urinary catecholamine levels as well as premature ventricular contractions on 12-lead ECG.38
Beta-blockers have been found to have a beneficial effect. Carvedilol 50 mg/day increased LVEF when it was <45 %.37 Metoprolol 50 mg/day induced improvement in clinical status, decreased noradrenaline serum levels, and increased systemic arterial pressure and LVEF on echocardiography.39 A sub-analysis of a prospective randomised trial performed on 25 patients showed increased survival in patients with Chagas heart failure.40 A longitudinal cohort study has demonstrated that metoprolol or carvedilol, even in small daily doses (carvedilol >9.75 mg), are associated with improved survival.41 Finally, the use of beta-blockers may have been responsible for the decrease in mortality observed in patients with Chagas heart failure in recent years.42 When the combination of ACEIs and beta-blockers provokes symptomatic systemic arterial hypotension, and the target dose of each agent cannot be reached, it has been suggested that the ACEI should be decreased and the target dose of beta-blocker be given.9
Digoxin produces a decrease in renin–angiotensin system activity43 and an improvement in haemodynamic profile44 when administered acutely to patients with Chagas heart failure. Nonetheless, the chronic use of digoxin is an independent predictor of mortality in such patients,34 probably due to digoxin intoxication. Increased digoxin levels have been detected in about 55 % of patients with Chagas heart failure;45 therefore serum levels should be measured when digoxin is given to patients with this condition.
It is important to emphasise that the suggestions for treating patients with Chagas heart failure with diuretics, mineralocorticoid antagonists, ACEIs, beta-blockers and digoxin are in line with the European Society of Cardiology guidelines for the treatment of patients with non-Chagas heart failure.46
Aetiological treatment with benznidazole has no effect in patients with mild Chagas heart failure. In the BENznidazole Evaluation For Interrupting Trypanosomiasis (BENEFIT) trial, which included about 2,854 Chagas disease patients – 17 % of whom had left ventricular systolic dysfunction – benznidazole showed no benefit over placebo in terms of morbidity and mortality. Aetiological treatment should not therefore be given to patients with Chagas heart failure.47
Non-surgical Device Therapy
SCD occurs in about 46 % of patients with Chagas heart failure.25 In >90 % of cases, SCD is caused by ventricular tachycardia degenerating into ventricular fibrillation or direct ventricular fibrillation.8 These conditions have a prevalence of 20 % in patients with severe Chagas heart failure (LVEF <35 %).48 Cardinalli-Neto et al.48 observed that patients with Chagas heart failure have a higher incidence of repetitive ventricular tachycardia and/or ventricular fibrillation than those with non-Chagas heart failure.48 These repetitive arrhythmias lead to shock occurrence in primary SCD prevention with an implantable cardioverter defibrillator (ICD). Shock occurrence was appropriate in all situations, affecting about 23% of patients; all Chagas heart failure patients could therefore be considered for ICDs.49
Amiodarone therapy has been associated with an unfavourable prognosis in patients with Chagas heart failure;50 moreover, in patients with mild Chagas heart failure (LVEF 45.8 ± 11.2 %) amiodarone is an independent predictor of inducible ventricular tachycardia on electrophysiological study.51 Patients with Chagas heart failure and a LVEF <35 % may therefore be suitable for primary prevention of SCD because their clinical profile is similar to that in patients with non- Chagas heart failure suitable for ICD treatment.
ICDs also appear to be of value in the secondary prevention of SCD. The number ICD shocks and episodes of ventricular tachycardia or ventricular fibrillation are higher in Chagas disease than in non-Chagas disease patients with milder forms of CHF.52 ICD implantation has shown that the risk of arrhythmia recurrence is high in patients with mild CHF;30,53 moreover, left ventricular diastolic diameter, a marker of SCD,25 is also a predictor of appropriate shock therapy in patients with mild CHF secondary to Chagas cardiomyopathy.54 ICD therapy also seems to decrease the number of SCDs in patients with mild Chagas heart failure.55 In a cohort of patients receiving ICD therapy compared with historical control patients taking amiodarone, ICD decreased all-cause mortality and SCD in patients with this condition.56 Thus, physicians dealing with patients with Chagas heart failure and ventricular tachycardia/ventricular fibrillation should refer such patients to receive ICD therapy for the secondary prevention of SCD.
The efficacy of cardiac resynchronisation therapy (CRT) in the setting of Chagas heart failure is not completely clear in view of the paucity of studies. Silva et al. studied the effects of CRT in patients receiving right ventricular pacing and observed an increase in LVEF and an improvement in functional status.57 Araujo et al.58 reported an increase in LVEF and a decrease in left ventricular systolic volume. The European Society of Cardiology guidelines have recently recommended CRT for non-Chagas heart failure patients with a QRS duration >150 msec and a non-LBBB. These guidelines also recommend patients with non-LBBB and a QRS duration between 130 and 149 msec be considered for CRT.46 Taking into account that about 16 % of patients with Chagas heart failure will have LBBB, almost half of them will have non-LBBB17,18,21 and the beneficial effects obtained with CRT in such patients make it reasonable to suggest CRT for selected patients with this condition.
Heart Transplantation
The prognosis for Chagas disease patients on the heart transplant waiting list is worse than that of non-Chagas disease patients.59 Heart transplantation (HT) is the treatment of choice for patients with this condition. Patients with an annual mortality higher than around 70 % should be considered for HT. Such patients are those with persistent NYHA Class IV, LVEF <30 %, on inotropic support, that have a maximal oxygen consumption rate <10 ml/kg/min, electric storm, hyponatremia along with LVEF <31 %, are not taking beta-blockers and those using digoxin.60
Aetiological treatment for HT candidates with Chagas disease is not usually recommended because it is ineffective in precluding the reactivation of acute Chagas disease in the HT recipient.61,62 In the case of inadvertent HT from an infected to an uninfected patient, however, the case is different. Blood and tissues should be closely monitored for signs of the parasite for at least for 2 years post transplant and, if found, aetiological treatment should be started as soon as possible.63
Morbidity in the perioperative period is similar to that found in non- Chagas disease centres in Brazil. Mortality in Brazil varies from 9 to 22 % because of the widespread unavailability of mechanical circulatory support;60 however a study of 11 patients in the United States showed a 6-month mortality of 18 %, consistent with results from South America, which is not significantly different.64
With the exception of potential reactivation of the disease, the posttransplant complications (rejection, infection, neoplasia and cardiac allograft vasculopathy) in Chagas HT recipients are similar to those found in non-Chagas HT recipients.60 In Bestetti et al.’s experience, the incidence of infection is lower in Chagas than in non-Chagas HT recipients.61
T. cruzi infection reactivation prevalence varies from 27 to 90 %, with a mean prevalence of around 35 %, and can be confounded with rejection in about 43 % of cases. Reactivation can be found from 1 to the 24 months post-HT.65 Mycophenolate mofetil appears to be a risk factor for T. cruzi reactivation.61,66 Reactivation is treated with benznidazole or nifurtimox, and mortality is very low (0.7 %),62 even in cases with central nervous system involvement.67 Clearly, HT is a formal indication for the treatment of end-stage Chagas heart failure.
Cell Therapy
A randomised trial of intracoronary injection of autologous bone marrow-derived mononuclear cells versus placebo in patients with severe Chagas heart failure has shown no benefit in comparison to placebo.68 This therapy should therefore not be given to patients with this condition.
Conclusion
Chagas heart failure has differences in clinical features, subsidiary tests and course that distinguish it from non-Chagas heart failure. The treatment, however, is similar to that available for non-Chagas heart failure. Physicians dealing with this disease should be aware of the differences in presentation and similarities in treatment to ensure patients with this condition are correctly diagnosed receive the best treatment.