The use of left ventricular assist devices (LVADs) in patients with advanced heart failure is well established; they make up 90% of all implanted continuous flow ventricular assist devices (VADs).1 In advanced heart failure, they are superior to medical therapy alone,1 with improvements demonstrated in survival, quality of life and functional status in carefully selected patients.2,3 Although initially designed as a bridge to cardiac transplantation, they have been reportedly used as destination therapy in 46% of cases, while 23% are implanted in anticipation of potential cardiac transplantation (bridge to candidacy).1
A significant limitation of LVADs is the lack of corresponding right heart support, which results in one-third of patients developing clinical right heart failure after LVAD implantation.4,5 Because there was some early success in these very sick patients, studies have evaluated applicability of VADs in a biventricular configuration (dual LVAD therapy) – one to support the left ventricle (LV) and another to support the right ventricle (RV).6–10 Compared with traditional pulsatile biventricular assist device (BiVAD) systems, dual VAD therapy has demonstrated improved survival rates and fewer adverse events, and enabled hospital discharge.6–10 Despite this, better outcomes continue to be achieved with isolated LVADs, producing an environment where durable BiVAD are only undertaken in irretrievable cases.1,12
To date, biventricular support has been offered to patients unsuitable for isolated LVAD therapy who have pre-existing, right heart failure or impairment and who are thought unlikely to improve after LVAD implantation.
It is important to recognise that those requiring biventricular support are a different population from patients who undergo univentricular support. These patients are generally sicker, with more profound multiple organ dysfunction than those requiring LVAD. Implanting LVADs into a patient with concomitant right ventricular failure (RVF) or at risk of developing RVF is associated with high mortality.4
With many durable BiVAD systems under development and testing, establishing clear criteria for LVAD and BiVAD support is imperative in providing optimum patient care. Therefore, early intervention, effective patient selection and sound criteria for device selection are required for making the decision between univentricular versus biventricular support.
Patient Selection
Patient selection involves identifying suitable candidates who have a minimum risk of developing adverse events while receiving the maximum benefits of VAD support.
Ideally, a universal selection criterion based on validated research should be used to ensure a standardised process of identifying suitable candidates for VAD support. This would simplify the selection criteria and enable correlation between different studies and centres. However, to date, no such standard exists. While few clinical trials are available to determine the general requirements of mechanical support with LVADs, no such trials exist with continuous flow BiVADs, resulting in patients being selected depending on individualised institutional criteria, such as clinical status, inotrope dependence and invasive haemodynamic and echocardiographic parameters. Based on these variables, individual predictors of poor operative outcomes for both LVAD and BiVAD support have been derived, including age, female sex, RVF markers, respiratory failure, impaired renal or hepatic function, diabetes, prior cardiac surgery, preoperative extracorporeal membrane oxygenation use, infection and coagulation abnormalities such as a declining platelet count.13–20 With a few key exceptions, most of these predictors are common in patients suitable for both LVAD and BiVAD support, which makes deciding between univentricular and biventricular support all the more challenging.
Patient Selection for Left Ventricular Assist Devices
LVADs are used in patients with advanced heart failure who are at a high risk of cardiogenic shock and/or multiple organ failure. The objective is to improve circulation, alleviate symptoms, improve quality of life and bridge suitable candidates to transplantation. Additional benefits of LVAD support include stabilisation or reversal of left ventricular dysfunction or pulmonary vascular hypertension.
Factors to consider when selecting patients for LVAD support include inotropic dependence, maximally tolerated medical therapy, low LV ejection fraction (<25%), declining renal function or low systolic blood pressure (<80 mmHg) or high pulmonary capillary wedge pressure (>20 mmHg).21 These variables are well-recognised clinical signs of LV failure.
However, rather than a focus on individual predictors, a more holistic approach of the patient’s clinical condition should guide the decision. This includes identifying potential contraindications for support such as evidence of aortic regurgitation, severe comorbidities such as renal or liver failure that are unlikely to be salvageable, active infection and/or bleeding, low platelet count, existing LV thrombus, psychosocial limitations such as the inability to comply with medical regimen or device maintenance, and a pre-existing high risk of developing RVF. Recently, it has been increasingly recognised that physical and cognitive frailty are strong predictors of outcome after LVAD implantation, and efforts have been made to define and standardise the assessment of these more accurately.22
Impact of an Left Ventricular Assist Devices on the Right Ventricle
The development of RVF is multifactorial and may not become apparent until after LVAD implantation. A healthy RV is responsible for delivering blood through the lungs and to the left ventricle. The inability to perform such an action can result in inadequate blood delivery to the left ventricle and therefore a decreased delivery of oxygenated blood to the tissue and organs.23 While the LVAD can improve left ventricular activity, it does not directly aid the RV to pump blood to the lungs. However, the LVAD can indirectly influence the RV by affecting factors such as venous return, septal motion and pulmonary artery pressure.24
In severe secondary pulmonary hypertension, the use of an LVAD has been shown to relieve pulmonary pressure and decrease right ventricular afterload.25 This is achieved by increasing forward flow to the body, thereby decreasing right ventricular afterload. However, the increased cardiac output created by the LVAD may overwhelm the load-sensitive RV. Moreover, by decreasing intraventricular LV pressure, the use of the LVAD forces the wall of the septum to shift towards the left, with altered RV geometry resulting in RVF because of decreased RV contractility and reduced RV compliance.24
Patient Selection for Biventricular Assist Devices
The decision to implant BiVADs can be difficult and varies between institutions. The main indications for early biventricular support have been acute circulatory collapse due to fulminant myocarditis, acute decompensation of dilated biventricular cardiomyopathy, massive MI (involving the septum or RV) or acute deterioration following toxic cardiomyopathy. BiVAD support may be more suitable in the presence of other widespread cardiac pathologies such as infiltrative cardiomyopathy or RV cardiomyopathies with concomitant involvement of the LV.7,26
Predicting RVF can improve patient and device selection and significantly affect patient outcomes. As such, numerous studies have closely examined the performance and structural integrity of the RV to find potential predictors of RVF. Echocardiographic and haemodynamic markers of RVF include: severe tricuspid regurgitation; low RV ejection fraction <30%; right atrial (RA) diameter >50 mm; decreased right ventricular stroke work index; markers of elevated serum bilirubin and creatinine; pulmonary artery pressure or elevated central venous pressure, especially relative to LV filling pressures with systemic hypoperfusion.17,18,27–31 While cut-offs for these variables differ between studies, increased research in the area can gradually lead to standards being established that can be adapted in future studies.
Unlike with LVADs, no clinical trials have been conducted to determine risk scores for patients in need of BiVAD support. Given the proven difficulty in assessing patients at risk for RVF clinically, attempts have been made to develop scoring systems, incorporating clinical, echocardiographic and haemodynamic markers.31–34 Despite the appeal of these apparently objective assessments, the predictive capacity of any one of them is limited outside its derivation cohort. The most recent appears to have some promise and represents the largest derivation and validation cohort to date.35
Time of Intervention
In addition to selecting the correct patients for isolated LVAD or BiVAD support, great consideration must also be given to selecting patients with sufficient severity of illness to achieve a benefit while avoiding those who are too ill or too early in the disease course to derive any gain.
VADs are often considered after all other therapeutic options such as lifestyle changes and pharmacological interventions have failed to restore a patient’s cardiac functional status. At the end-stage period of the disease, patients are often plagued with various comorbidities and have significant limitations because of poor circulation. Although VADs can restore circulation, their efficacy is limited by the patient’s comorbidity profile and clinical status. The timing of device intervention is therefore crucial in achieving optimum outcomes.
There is a general consensus that patients receiving an implant earlier in the clinical course of their disease perform better in terms of survival than those having the procedure at the end stage. This can be seen in the seventh annual report of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACs), where INTERMACs groups 3 and 4 have higher survival rates than INTERMACs groups 1 and 2.2 Similarly, there is clear evidence to associate early BiVAD support with superior outcomes compared to delayed RVAD support following LVAD implantation.36,37 The disadvantage of delayed RVAD support is that an already compromised patient must undergo an additional surgical procedure. Moreover, patients who require delayed RVAD support are by definition failing isolated LVAD support and therefore have worse haemodynamic instability and circulatory flow than someone without a need for subsequent RVAD support.
Takeda et al. assessed the impact of initial versus delayed RVAD support using the Levitronix CentriMag and showed that initial RVAD support resulted in significantly improved survival and transplantation rates compared to delayed RVAD support.37 These findings are also supported in recent durable BiVAD studies, which strongly favoured planned BiVAD support.7 While BiVAD recipients have greater mortality and morbidity than LVAD recipients, survival following cardiac transplantation does not appear to be associated with the type of configuration used (LVAD versus BiVAD).38 Therefore, as long as BiVAD recipients are successfully bridged to cardiac transplantation, they will have similar post-transplant survival rates as their LVAD counterparts. As the pathology of biventricular failure is largely associated with poor systemic blood circulation, it is logical to assume that early intervention with durable BiVADs can reduce mortality rates during the early postoperative period and therefore increase the number of successfully bridged candidates. The challenge then will be to determine the difference between early and too early.
Device Selection
The type of device, its weight, size, flow dynamics (pulsatile versus continuous) and mode of support (extra- or para-corporeal or implantable) can all affect its suitability as a biventricular system. Continuous flow miniaturised devices have an advantage over larger extra/paracorporeal pulsatile flow models as they require less surgical invasiveness so a shorter procedural time. Continuous flow VADs are also mechanically simpler, with better device durability.
The surgical procedure and implantation technique can also influence device selection. For example, despite successful implantation and RVAD support with the continuous flow Jarvik 2000 implantable LVAD system, the connection to the descending aorta has made its removal during subsequent heart transplantation difficult, extending both surgical and cardiopulmonary bypass time.39
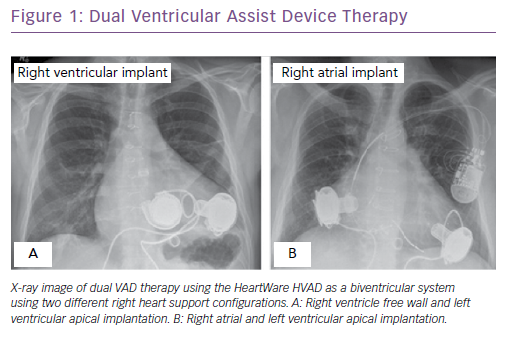
Other devices, such as the HeartWare HVAD, can be used for RV support but require minor modifications to implantation technique.6,7,9 The HVAD inflow cannula is too long for the dimension of the RV and, if unaddressed, this may lead to interaction with the interventricular septum or other chambers of the heart, resulting in obstruction and reduced circulatory flow. To circumvent this, it has been suggested that spacers are placed between the RV wall and the HVAD to decrease the protrusion of the inflow cannula into the RV or RA cavity. This reduces the risk of RVAD flow obstruction and thrombus formation. In addition, to reduce the risk of pulmonary oedema caused by high RVAD flows to the lungs, banding of the outflow graft may be performed to increase resistance. However, in the event of RV recovery, the RV dramatically shrinks due to structural remodelling and draws the inflow cannula closer to the interventricular septum, increasing the risk of occlusion and potentially promoting thrombus formation. As a result, alternative implantation sites have been investigated such as the right atrium (Figure 1).7 Right atrial implantation reduces the risk of occlusion from interactions with the interventricular septum. It also eliminates the need for additional surgical correction in the presence of severe tricuspid regurgitation and potentially increases right ventricular cardiac output in such patients. Additionally, the RA site enables implantation in smaller patients without risk of VAD positional changes following sternum closure.7 Implantation of a second VAD into right heart chambers remains an unapproved indication by regulatory authorities.
The HVAD is capable of providing long-term BiVAD support with favourable outcomes. Ideally, early planned BiVAD support should be considered in patients with concomitant RVF. However, in those with mild RVF who are likely to recover, long-term durable RVAD support may not be the optimum choice. To date, few options are available for short-term RV support. The Levitronix CentriMag is an extracorporeal, third generation, continuous flow system that has successfully provided short term RVAD support (Figure 2).37,40,41 RVAD support is commonly established with the RA as inflow and the pulmonary artery as outflow. The advantage of the Levitronix CentriMag are its lack of bearing and seals, which reduces thermal damage to blood component and haemolysis, as well as the risks of thrombus formation and mechanical failure. In addition, it can be implanted in smaller patients, such as women and children. Its application as a RVAD has yielded variable outcomes with 30-day survival rates in the 30–75% range.40,42,43 Major complications associated with the CentriMag include bleeding, infection, respiratory failure, liver dysfunction and neurological complications.40,42,43
Short-term RVAD support has been successfully performed with various VAD models yielding similar results to long-term BiVAD outcomes.37,44 Technological advances in venopulmonary arterial extracorporeal life support (VPA–ECLS) have also made it a viable option for temporary RVAD support, warranting further investigation. These short-term devices can successfully unload the RV during the perioperative period, stabilise RV parameters, transition patients back on isolated LVAD support and eliminate the consequences of long-term BiVAD support. The greatest inherent advantage of VPA–ECLS devices compared to RVAD systems is that they can be implanted at the bedside, provide concomitant oxygenator support and can be removed without reoperation, reducing the risk of additional open heart surgery. Oxygenators can be useful in the event of severe lung oedema, hypoxia or in patients on ECLS support prior to receiving VAD support.45 While promising, survival rates with more durable RVAD support systems remain similar to those with BiVAD support.46,47 Nonetheless, owing to the novelty of these interventions, improvements in survival and wean rates can be expected with improved patient selection and postoperative management.
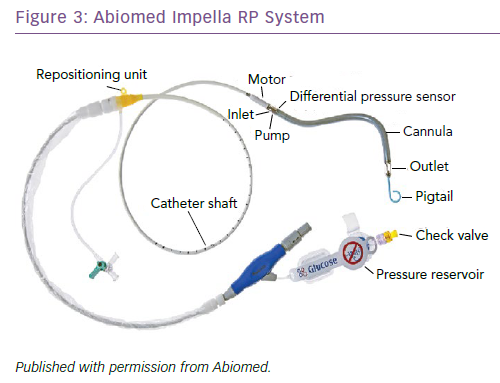
Another short-term RVAD option is the Abiomed Impella RP Heart Pump (Impella RP), which uses a 22 Fr catheter-mounted microaxial flow pump (Figure 3). It is designed to be implanted percutaneously, using a standard catheterisation procedure via the femoral vein. Blood enters the microaxial pump via the inflow cannula positioned at the inferior vena cava and is delivered directly to the pulmonary artery at a rate of up to 4 l/min, bypassing the RA and the tricuspid and pulmonary valves. The Impella RP is relatively new, and few single-centre studies have reported on outcomes. The prospective RECOVER RIGHT study assessed the safety and benefits of the Impella RP in RVF patients after LVAD implantation as well as after a cardiotomy.48 The 6-month survival rates were better in the post-LVAD group at approximately 80% compared to approximately 60% in those who had had a cardiotomy. Although the device is approved for 14 days of temporary RV support, patients required support for an average of only 4 days before successful RV haemodynamic stabilisation and subsequent weaning.48 The main advantage of the Impella RP is its simple implantation procedure, which allows rapid intervention before RV shock becomes irreversible. There are contraindications, however, which include a body surface area <1.5 m2, the presence of mechanical valves, severe valvular stenosis or valvular regurgitation of the tricuspid or pulmonary valve, and other disorders of the pulmonary artery wall that would preclude placement or correct positioning of the Impeller RP device. Although small studies and anecdotal cases have demonstrated positive outcomes, further investigation is warranted.48–50
Conclusion
Deciding between univentricular and biventricular support remains complicated. This requires a holistic approach rather than considering isolated markers. Patients should be thoroughly screened and appropriately matched to the device that can yield optimum outcomes.
Patients with evident RVF may be considered for long-term dual VAD therapy for biventricular support. Those with mild signs of RVF may benefit from right-sided temporary percutaneous implantable pumps in the early perioperative period to allow for RV haemodynamic stabilisation.
Where weaning is impossible, temporary BiVADs can serve as a bridge to decision or potential cardiac transplantation in eligible patients.
Early intervention, careful patient and device selection can further improve outcomes in patients in need of BiVAD support and may help bridge the disparity between uni- and biventricular outcomes.