A hallmark symptom of chronic heart failure (CHF) is exercise intolerance associated with early fatigue and/or dyspnoea with a minimal degree of exertion. It is also associated with a decline in capacity to perform activities of daily living and a diminished quality of life (QoL). Both patients with heart failure with reduced left ventricular ejection fraction (HFrEF) and those with heart failure with preserved ejection fraction (HFpEF) have poorer prognostic risk factors, including increased mortality.1
In patients with CHF and persistent AF, exercise performance tends to be more impaired than it is in people in sinus rhythm. This is typically reflected by a decreased peak oxygen uptake (VO2), e.g. 13.4 ml/kg/min in patients with CHF and AF versus 15.2 ml/kg/min in patients with CHF in sinus rhythm.2,3 Concerning exercise performance, lower peak VO2 is an independent predictor of AF, but not the ventilation over carbondioxine (VE/VCO2) slope.
Exercise training to increase exercise capacity is important in the management of CHF and could counteract many negative consequences of AF. Moreover, a recent observation from the Women’s Health Study shows that in women with recent AF, risk factors such as obesity, hypertension, smoking and diabetes are predictive for CHF later in life. Countering these risk factors with appropriate therapy such as exercise training, smoking cessation and control of hypertension in AF could help to prevent the development of CHF.4,5
The general approach to AF management does not differ between people with CHF and other patients, but a few considerations are worth making.6
In this review, we seek to provide practical guidance on cardiac rehabilitation in patients with CHF and AF.
AF in Chronic Heart Failure: Prevalence and Prognosis
AF is the most common sustained clinical arrhythmia, with higher incidence and prevalence rates in developed countries, occurring in approximately 3 % of adults aged ≥20 years.6–9 The prevalence of AF rises markedly with age; many modifiable and non-modifiable risk factors underlie AF, including hypertension, CHF, coronary artery disease, valvular heart disease, obesity, type 2 diabetes, cardiomyopathies, congenital heart defects, long-term endurance exercise or chronic kidney disease.6,7,10–12
AF, especially when persistent or permanent, is present in at least 20 % of patients with CHF, with prevalence increasing with severity of the syndrome.13,14 It is associated with a poorer prognosis in patients with than those without CHF,and among patients with a higher incidence of more severe cardiac arrhythmias.1,15
CHF and AF share a common pathophysiology and can exacerbate one another through mechanisms including structural cardiac remodelling, activation of neurohormonal mechanisms and rate-related impairment of left ventricular function.16,17 Since CHF and AF often go hand in hand,AF will eventually occur in most people with CHF, particularly among older patients with HFpEF.18,19 Moreover, epidemiologic data suggest that people with AF have a 10-fold higher risk of developing CHF than those without AF.
While the focus of AF treatment usually involves ablation and optimising pharmacological therapy, these approaches could be insufficient to completely treat this condition. Holistic management of the patient including adequate CHF prevention is needed.19
Exercise Training in Patients with Chronic Heart Failure and AF
Physiology of Exercise Training
An exercise training programme for healthy individuals improves both central and peripheral determinants of the Fick equation – peak oxygen uptake (VO2) = cardiac output (CO) x arteriovenous O2 difference (a–vO2) – and therefore improves cardiac as well as skeletal muscle function.20
A recent review stated that peak VO2 is approximately 35 % lower in patients with CHF than in healthy subjects, with similar magnitudes of impairment in patients with HFrEF and HFpEF.21 Moreover, AF is associated with lower exercise capacity in both HFrEF and HFpEF.2,3,22 A landmark study of 1,744 patients diagnosed with HFpEF,of whom 239 showed AF, revealed that peak VO2 was significantly lower – 1.8 ml/kg/min – when AF was present.22 The authors observed that AF was associated with exercise intolerance, increased mortality and impaired contractile reserve.
Stroke volume is generally not modifiable with training in CHF and therefore a higher heart rate (HR) may occur in some patients to augment cardiac output.23 Particularly in HFpEF, exercise capacity appears to be largely mediated by inadequate blood flow to the active skeletal muscles secondary to impaired cardiac output.21
In both, HFpEF and HFrEF (particularly the former), the role of peripheral factors such as endothelial function, ergoreflex activation and vasodilatory capacity are important factors underlying exercise intolerance.21,24–26 In these patients, strength training in particular could be effective. In general, the combination of aerobic and strength training will positively modify these central and peripheral determinants of peak VO2.27–30
Clinical Evaluation Before Exercise Training
Before cardiac rehabilitation, patients with CHF require a clinical evaluation, optimal pharmacological therapy, risk stratification and treatment of the underlying causes of the condition.31 In addition, the numerous haemodynamic abnormalities in CHF should be considered as they underlie reduced exercise capacity and can therefore be improved by exercise training. Clinically stable patients with CHF are excellent candidates for cardiac rehabilitation. CHF stability should be verified based on daily changes in body weight, symptoms and comorbidities.
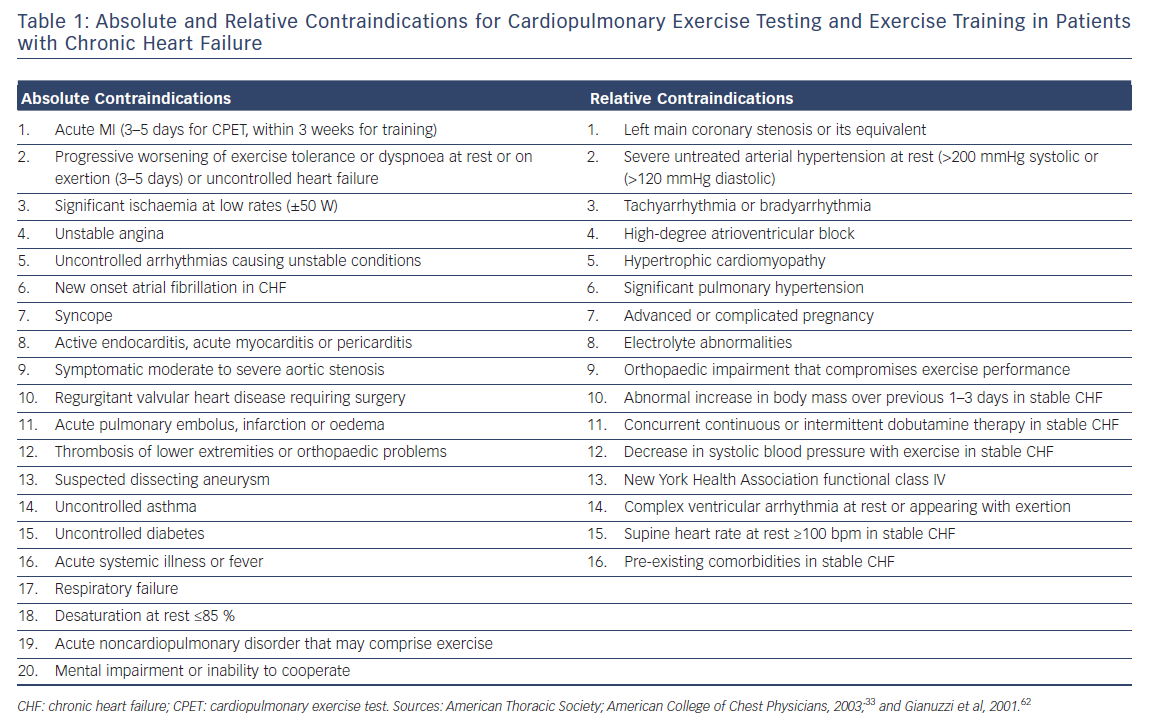
Before initiating a cardiac rehabilitation programme, a cardiopulmonary exercise test (CPET) with gas exchange analysis should be performed to evaluate safety, exercise capacity and prognosis.32 During a CPET, ECG monitoring. blood pressure recording and, where appropriate, O2 saturation should be performed. Relative and absolute contraindications for CPET and exercise training (Table 1) should be considered.33
There is no fundamental difference between performing a CPET and initiating cardiac rehabilitation for patients with CHF and AF and those with CHF alone. Although CPET assessment is considered to be the gold standard method for the evaluation of fitness and the function of the cardiorespiratory and muscular systems, in many centres, a graded exercise test is not usually completed with cardiac rehabilitation. If this is the case, alternative assessment methods can be used to obtain the intensity for rehabilitation. These are discussed below.
It is important to prescribe exercise in an individualised manner focusing on exercise capacity, QoL, activities of daily living and secondary prevention, as suggested by the International Classification of Functioning, Disability and Health.34 There is no single programme that is best for all patients or even one patient over time.31 Therefore, the clinician should evaluate the patient’s psychosocial, pathophysiologic, environmental and vocational factors and tailor them to the person’s needs and realistic goals. Selecting activities that the patient enjoys is likely to lead to better adherence to physical activities once the rehabilitation programme ends. Involving the patient’s family and taking into account his/her social activities tends to strengthen motivation and increase adherence to the exercise training programme.35 Moreover, the clinician should consider comorbidities, such as respiratory disease, diabetes, obesity and musculoskeletal disorders, when prescribing exercise as they can limit exercise performance.
The effect of training is best measured by peak VO2 as it reflects the capacity of the body to transport, use and distribute oxygen. Other criteria to assess training effects can include submaximal performance testing, the ability to perform activities of daily living, improvements in independence and being able to continue working, participate in social activities and the like. These important changes can occur without a significant increase in peak VO2.
Intensity, Duration, Frequency and Modality of Training
It should be noted that a fundamental prerequisite for exercise in patients with AF is appropriate HR control, not only at rest but also during exercise. It is beyond the scope of this review to thoroughly discuss the benefits and risks of rate and/or rhythm control in AF. Instead, this review focuses on training principles for patients under optimised pharmacotherapy. Moreover, prior research has shown that more lenient HR control (i.e. accepting rates at rest of up to 110 bpm) does not change outcome compared to more stringent HR control, and is associated with a reduced need for pacemakers.36,37 On the other hand, lenient HR control at rest can lead to rapidly conducted AF which will affect left ventricular function during exercise both acutely and possibly in the long term. In some patients, European Society of Cardiology guidelines recommend a strategy of HR control drugs, possibly combined with a pacemaker, to ensure HR is well controlled under different circumstances, including rehabilitation.38
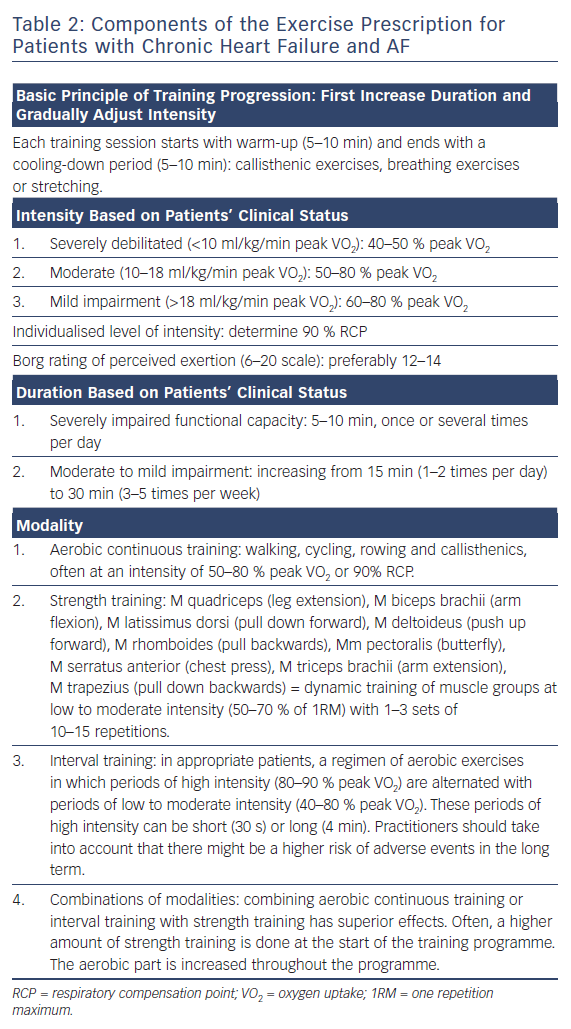
Intensity, duration, frequency and modality are the main elements of a training programme.30 The ways in which they are applied depend on the patient’s clinical status (Table 2). A training session should start with an appropriate warm-up (e.g. ~10 minutes of callisthenic exercises) and end with a cooling-down period (e.g. ~10 minutes of stretching and breathing exercises). In general, improvements in aerobic capacity are achieved if the patient exercises dynamically (employing large muscle groups using a treadmill or arm ergometer, cycling, stepping or rowing) for at least 30 minutes, three to five times per week at an intensity of 40–80 % of peak VO2.30 This range depends on the exercise modality, i.e. the time period in interval training or continuous training. In patients with stable CHF, training at 85–95 % peak VO2 has been said to be optimal.39 It should, however, be questioned if performance at these high percentages can be conducted effectively by individual patients considering influencing factors such as motivation and anxiety.
It is appropriate to start at a lower intensity for short periods and steadily increase duration and intensity throughout the rehabilitation period (often 3–6 months). The biggest challenge related to exercise prescription is individualising training intensity.
To optimally estimate individualised training intensity, peak VO2 should be measured during CPET. The patient then exercises at a percentage of the individual peak VO2 at the corresponding HR. For example, a patient who starts an exercise training programme at 50 % of peak VO2 should exercise at the corresponding HR, typically within a HR range at this intensity ±5 BPM.
Another objective method for individualising training intensity involves adopting an intensity commensurate with the respiratory compensation point (RCP) or second ventilatory threshold (VT2).40–42 With increasing exercise intensity and lactic acid production above the first ventilatory threshold (VT1), a point is reached when intracellular bicarbonate is no longer able to adequately counteract exercise-induced metabolic acidosis. At this point, respiratory alkalosis develops through a VE increase in excess of VCO2, and this is termed VT2 or RCP. Simultaneously, the VE/VCO2 ratio inverts its trend (it increases versus initial decrease), and the VT2 is identifiable as the nadir of the VE/VCO2 versus workload relationship.43 This point is defined as the exercise level at which ventilation increases exponentially relative to the increase in VCO2. At the RCP, the body cannot transport enough oxygen to compensate for the accumulating CO2 levels, lactate accumulates and hyperventilation occurs.42 When identifiable, the VT2 is usually attained around 70–80 % peak VO2 and 80–90 % peak HR reached during incremental exercise; it has been suggested that this is related to so-called “critical power”,that is, the upper intensity limit for prolonged aerobic exercise.44–46 CP presents the highest workload sustainable when both VO2 and lactate remain in a steady state.47,48 Independent of peak VO2, the RCP can occur earlier or later, depending on individual efficiency in oxygen delivery and the ability of the body to remove lactate. Because it is difficult to exercise at or beyond the RCP, an individualised training intensity of 90 % RCP is often implemented.42
Independent of the reference value – % peak VO2 or % RCP – it should be noted that in patients with CHF and AF (an estimated 10–30 % of patients with HF), the variation in HR is usually high and a fixed HR for a certain workload often cannot be measured accurately.49 Therefore, during CPET, the HR for a certain workload must be determined using a longer sampling interval (e.g. mean HR for 30 seconds at a given intensity for instance a 50 % peak VO2 or at 90 % RCP).
During “constant pulse rate” training, practitioners should be aware that not all equipment or training devices register the HR correctly during exercise when AF is present, since they do not average the HR over a longer sampling interval, for example a period of 1 minute. So, to exercise patients with CHF and AF at a constant pulse rate, telemetry should be used continuously throughout training sessions. If telemetry is not available in the rehabilitation facility, other options can be considered to rehabilitate these patients in a safe, individualised and effective manner. In these circumstances, training at “constant workload” associated with the load at 50 % peak VO2 or 90 % RCP is advised rather than the concomitant HR. The desired effect is that exercise training delays the occurrence of the RCP and therefore HR will be lower and workload will be higher at the RCP. Deciding an appropriate, individualised training intensity is more challenging when CPET is not available, but intensity can be determined using a percentage of maximal HR achieved, or what is known as the HR reserve or Karvonen formula (maximal HR − resting HR × desired intensity + resting HR). These methods are only reliable in patients in normal sinus rhythm whose measurements of resting and maximal heart rates are precise.
More subjective methods include estimation of the perceived exertion using the Borg scale. An intensity at a level between 12 and 14 on the 6–20 scale has been shown to be well tolerated and associated with favourable training responses.50,51 The “talk test”has also been suggested as a valid method to monitor exercise intensity when CPET assessment is not possible.52 It has been suggested that the 6-minute walk test is a simple and reliable test to estimate exercise tolerance in patients with CHF; however, it is not a valid tool for prescribing exercise intensity.53,54
Despite relatively limited literature, available studies are consistent in showing that low to moderate intensity aerobic physical activity improves exercise capacity, QoL and performance in activities of daily living in patients with AF.55,56 However, some authors recommend training for patients with CHF at the RCP level (i.e. the upper limit of the moderate to high-intensity range) for periods of 15–30 minutes. While this represents a higher than conventional intensity, it has been demonstrated to be safe.42,57,58 Studies are inconsistent in terms of the effects of long-term vigorous endurance exercise training and high-intensity interval training since these forms apply intensities more than 80 % peak VO2 or >90 % RCP, and may be associated with an increased incidence of AF and higher risks.55,56 Given the variability in the ventricular response in AF, it has been recommended that exercise intensity should be targeted at 10 BPM below the HR associated with the referenced criteria.
Recently, the potential superior effects of high-intensity interval training was investigated in trials involving patients with CHF. Despite initial promising results, a more recent, larger trial indicated that high-intensity interval training did not have superior effects on exercise capacity than moderate continuous training.29,59 Moreover, it is suggested that training at high intensity for short periods might raise the risk of hospital readmission in these patients. The question remains whether single bouts of exercise at high intensity are useful, safe, motivating for patients, and practical in CHF. A recent meta-analysis comparing modalities of exercise training in people with CHF (interval versus continuous training, with and without strength training) indicated that no training mode was superior to another in terms of prognosis, exercise capacity and QoL, even though left ventricular function appeared to show greater improvement with interval training.30
More research should be directed toward the most effective training modality in people with CHF. In patients with CHF and AF, implementing high intensities is not recommended at this time.
Resistance training was once thought to be strictly contraindicated in patients with CHF. However, it is now widely recommended to restore muscular strength. CHF is known to be a muscle-wasting disease and many affected patients experience weakness of the large muscle groups when they start a cardiac rehabilitation programme. Resistance training has been demonstrated to be safe and to increase exercise capacity (by facilitating peripheral oxygen extraction), reduce blood pressure and increase the capacity to perform activities of daily living.42,58,60 In cardiac rehabilitation programmes, strength training is often combined with aerobic training. As with aerobic training, resistance training should begin slowly and progress gradually according to a patient’s ability.58 Strength training should focus on the major muscle groups and one repetition maximum (1RM) should be tested at initiation. An appropriate technique in patients with CHF and AF might be 10–15 RM testing, which reflects the maximum weight that a person can move 10–15 times. One set of 10–15 repetitions at an intensity of 50–70 % of 1RM is usually appropriate at the beginning of the programme. Sets can then be increased from one to three throughout the period.58,60
Future Directions and Conclusions
Throughout the world, key cardiology organisations focusing on prevention have developed recommendations for cardiac rehabilitation programmes.61 Historically, these have focused on patients with coronary artery disease, and recommendations for patients with CHF have been made much more recently. Much less research has looked at other populations with cardiovascular disease; to the best of the authors’ knowledge, no recommendations have been made specifically for patients with CHF and AF. Clinical trials comparing exercise training modalities, intensities, frequency and duration for this specific population are lacking. Therefore, the practical guidelines discussed here are based on recommendations for patients with CHF and adjusted based on the available literature and the authors’ clinical experience. Further refinement of exercise training methods, along with evaluating the effects of training on prognosis and QoL in patients with CHF and AF are needed from future randomised trials.
In conclusion, an exercise training programme of moderate intensity is advised for patients with CHF and AF. Optimal clinical evaluation using CPET should be carried out before a training programme is started. In patients with CHF and AF, the average HR should be calculated over a longer time period than it is in patients in sinus rhythm. The use of telemetry is advised to measure HR accurately during training since most training devices do not estimate HR accurately in patients with AF. If telemetry is not available during training, patients can be safely trained based on the concomitant workload and perceived exertion.