Because of the ageing population in industrialised countries, the prevalence of cardiovascular disease has dramatically increased.1 In particular, heart failure (HF) has become a major public health problem and the leading cause of morbidity, hospitalisation and mortality in older people.2,3
Patients with HF, especially the oldest, are often characterised by a “vulnerability status” due to the presence of several comorbidities and impaired functional capacity.4,5 The Cardiovascular Health Study,6 a US longitudinal cohort of older people living in the community, has defined this vulnerability status as the “frail phenotype”. This term indicates elderly people with multiple chronic morbidities, resulting in reduced autonomy in daily life activities.6 Of note, the Cardiovascular Health Study found frailty was associated with clinical cardiovascular disease, most strongly with HF. Indeed, almost half of patients with HF were frail, and this was independent of age or New York Heart Association functional classification. The frail phenotype was defined using five criteria: unintentional weight loss; self-reported exhaustion; low energy expenditure; slow gait speed; and weak grip strength. The study also demonstrated the relationships between frailty, comorbidity and disability found to predict and/or exacerbate each other.
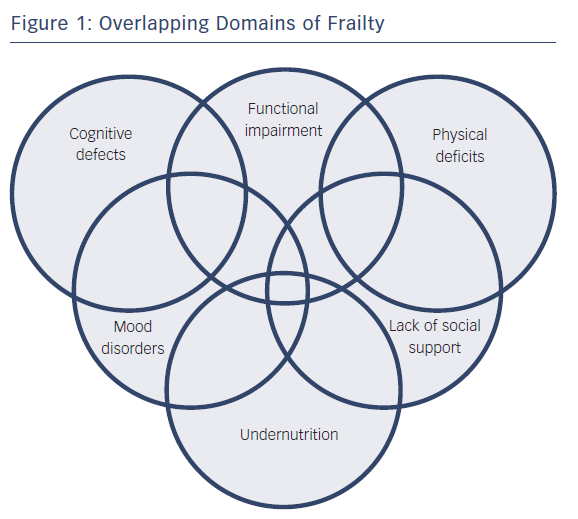
Although the precise pathogenesis of frailty in HF has not been fully elucidated, shared pathophysiologic mechanisms may help explain the complex relationships among frailty, comorbidity and disability.7 According to the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults,8 a “dependency cascade” may occur. This term indicates a progressive series of damage across multiple organ systems, ranging from functional decline to disability and death. The most common deficits relate to mobility, strength, balance, motor processing, cognition, nutrition, endurance and physical activity.9
Domains of frailty are shown in Figure 1. As deficits in these domains accumulate, the capacity to cope with distress is diminished, and functional decline worsens.10,11 This self-reinforcing dependency cascade may be a consequence of impaired homeostatic maintenance/repair mechanisms.12 Disorders in neurohormonal, metabolic, immunologic and musculoskeletal systems may lead to an increased catabolic state that is typical of the frail phenotype in HF.4 For these reasons, patients who are frail experience sarcopenia, tissue wasting and cardiac cachexia, with consequent weakness, fatigue and reduced resistance to stressors.13
Therefore, frailty in HF is characterised not only by myocardial failure but also by concomitant metabolic failure.4 Frailty and the above-mentioned associated conditions, i.e. cachexia, sarcopenia and reduced functional capacity, are particularly prevalent in patients with advanced HF.14 It still has to be clarified whether therapies aimed at addressing neurohumoral overactivation and improving haemodynamics and cardiac and muscular metabolism may be effective in ameliorating these conditions.
Prognostic Implications
Not only are elderly patients with HF at increased risk of developing frailty, but also frail older adults are more likely to develop new-onset HF.15 Therefore, the relationship between frailty and HF is bidirectional. This is thought to be due to the common underlying mechanisms of inflammation, metabolic dysfunction and hormonal dysregulation.16,17
Older adults with frailty and HF are at increased risk of poor clinical outcomes.15 Observational studies have found frailty to be associated with higher mortality rates and healthcare utilisation, including emergency department visits and hospitalisations, disability, falls and cognitive decline.15,18–22 In elderly patients with chronic HF, the presence of frailty was associated with an increased risk of mortality, hospital readmission and functional decline at one year.23
Furthermore, patients with HF and frailty have a worse quality of life since frailty accelerates the risk of developing a disability.6 The FRAIL-HF, a prospective cohort study including 450 non-dependent patients aged ≥70 years who had been hospitalised for HF,21 evaluated the relationships between the frailty phenotype and associated issues (i.e. comorbidities, coexistent geriatric syndromes, self-care and social support) with clinical, functional and quality-of-life outcomes. The study found that even in non-dependent patients, frailty was a risk factor for early disability, long-term mortality and hospital readmission.20
Assessment and Management of Frailty in HF
Recognising the frailty phenotype in patients with HF may help to detect those at risk of poor outcomes (i.e. disability, death, hospitalisation or institutionalisation)23,24 so identify those who need early intervention and close monitoring.25 The recognition of frailty is the first step for an accurate risk stratification and planning a tailored therapeutic plan.26,27
However, several knowledge gaps exist. First, a unique definition of this syndrome in patients with HF is still lacking.26 Second, most trials on HF have excluded elderly patients with comorbidities, who comprise the population at the highest risk of frailty.25 Third, an established frailty approach in HF is still missing.
Several measurements have been proposed to assess frailty, and a huge variety of methods and instruments are available.11,28,29 The most widely used tools to assess frailty are Fried’s phenotypic definition of frailty and the frailty index, described in detail elsewhere.3,11,30 There are also self-report questionnaires or instruments based on the assessment of single performances (i.e. single domains of frailty). However, a recent systematic review30 found inconsistencies in frailty measurements and identified that the only consistently represented domain across frailty instruments was physical function/mobility.
There is, therefore, a need for a consensus from the scientific community for a standard method – preferably user friendly and not time consuming – that can be used to accurately identify frailty in patients with cardiovascular conditions and reliably predict adverse clinical outcomes.
To date, the multidimensional interdisciplinary Comprehensive Geriatric Assessment (CGA) is the most used tool to measure frailty. However, this instrument may not adequately characterise frailty in patients with cardiac conditions. To date, there are no validated frailty instruments specific for HF or other cardiovascular conditions.31,32 A recent systematic review30 identified seven frailty assessment instruments that have been used in HF research so far. Of these seven instruments – the Frailty Phenotype, the Deficit Accumulation Index, the Tilburg Frailty Indicator, the CGA, the Frailty Staging System, the Canadian Health and Ageing Clinical Frailty Scale and the Survey of Health, Ageing and Retirement in Europe Frailty Index – none have been validated for use in HF. Assessing frailty with a validated instrument is a priority, international frailty guidelines recommend.33,34
An accurate assessment of frailty in older patients with HF should include its early identification, consideration of referral to specialists, such as cardiologists, primary care physicians and specialist nurses, and anticipation of care.9
The correct timing for the diagnosis of frailty in HF is yet to be established.35 A recent position paper from acute coronary care specialists suggests that, although challenging, the assessment of frailty should be performed during the acute phase of hospital admission.25 However, at this stage, several confounding factors may influence the detection of frailty. For now, self-reported assessments should be preferred, while objective performance measures may be used at discharge.25
In any case, routine assessment of frailty should be part of a holistic management plan in patients with HF.30
Finally, given that health status is reduced in patients with HF and frailty, measuring patient-reported health status is pivotal for cardiovascular conditions, according to the American Heart Association (AHA).36 A position paper from the AHA, American College of Cardiology and American Geriatrics Society states that future guidelines should take into account the assessment of frailty domains as well as chronological age in the management of older patients with HF.37 As most older adults are not frail, chronological age is not a reliable indicator of biological age and health status. Frailty has been found to be a better predictor of age-dependent heart rhythm disorders than age.38
A comprehensive approach to manage frailty in HF should therefore start from a multidimensional assessment.39 The domains should include mobility, strength, balance, motor processing, cognition, nutrition, endurance and physical activity.9,39 According to the European Society of Cardiology guidelines, the management of older adults with HF includes the monitoring of frailty over time, taking into account its reversible causes to prevent increasing frailty.40 In particular, optimisation of symptom control may improve exercise tolerance, with improvements in skeletal muscle function and sarcopenia, which ameliorate functional status.41 Exercise training may reduce the frequency of HF exacerbations and hospitalisations, and improve functional capacity, quality of life and survival in HF patients.42
Hormonal treatment with testosterone has been shown to improve functional capacity and quality of life in elderly patients with HF in both sexes but this therapeutic approach needs to be specifically tested in frail patients with HF.43,44
Therefore, frailty may be a dynamic state when its cardiovascular and non-cardiovascular causes may be reversed.40 In addition, rehabilitative programmes may be used to delay or prevent functional decline. In particular, exercise training has a positive effect on physical function and functional capacity in elderly people who are frail.45
In addition, frailty poses special challenges for patients needing end-of-life care. As reviewed in detail elsewhere,46 palliative care should be provided by an interdisciplinary team, with the aims of relieving symptoms (in particular, pain control) and offering psychological support to patients and caregivers to improve quality of life. Under-nutrition and unintentional weight loss are key issues for these patients.47 Therefore, correction of nutrition is a main component of frailty management, although it still remains to be clarified whether vitamin/mineral supplements and/or hormone supplements may be beneficial.45
Conclusions
Frailty is a dynamic and potentially reversible state. An accurate assessment may allow a tailored, individualised healthcare programme of management to prevent adverse outcomes in HF. Therefore, a consensual and multidimensional assessment is needed.
Future trials should include methods to select and stratify participants with frailty as they are an especially high-risk group.25 Finally, upcoming studies should aim to clarify an age threshold for subjects’ inclusion in future research.48
Managing frailty may help improve quality of life and have a substantial impact on prognosis in HF. Considering the role of frailty in clinical management of HF is therefore pivotal.