The use of IV vasoactive drugs, diuretics, vasodilators and inotropes for correcting haemodynamic dysfunction in patients with decompensated heart failure has been described over many decades.1 However, data on their effects on prognosis do not offer a convincing picture of clinical benefit.2 This is particularly true regarding IV inotropes. Clinical data collected on the effects of cardiac glycosides, catecholamines and phosphodiesterase inhibitors indicate an overall increase in mortality risk.3,4 Increased cardiomyocyte oxygen consumption in ischaemically jeopardised myocardium, plus a heightened propensity to cardiac arrhythmias, have been proposed as possible explanations for these findings.5
The calcium sensitiser and potassium channel opener levosimendan has emerged in recent years as potentially a safer inotropic option than the traditional classes of cardio-mobilising drugs by virtue of its different mechanism of action.6,7 Levosimendan delivers inotropy via a broadly energy-neutral route, and vasodilation, including reduction of central venous pressure, relief of hepatic congestion and indications of improvement in renal function.8 Taken in combination with an extended duration of effect ascribable to a long-acting metabolite, this profile identifies levosimendan as a unique inotrope for the management of acute heart failure (AHF) and advanced heart failure (AdHF).9–12
This article presents some views on the use of vasoactive drugs in the management of AHF and AdHF that emerged during a series of tutorials held in conjunction with the annual congress of the Heart Failure Association of the European Society of Cardiology (ESC), in Athens, Greece in May 2019. Twelve speakers (from Austria, Cyprus, Finland, Germany, Greece, Hungary, Italy, Spain, Sweden and Switzerland) delivered the tutorials and collaborated in the development of this text.
Levosimendan in Acute Heart Failure
The assessment and management of AHF have been set on a robust practical footing by the most recent ESC guidelines, to which readers are referred for a comprehensive statement on this subject.13 Summarising broadly, AHF may be described as a situation of rapid onset or worsening of the signs and symptoms of HF. AHF must, inter alia, be characterised as a life-threatening medical condition that requires urgent evaluation and management and frequently leads to hospitalisation.
AHF may present de novo or as a deterioration in chronic HF. Many cases will arise from primary cardiac dysfunction, notably MI, but extrinsic precipitants, such as infection or anaemia, may play a role, along with an extensive range of triggering factors.13 Other high-risk cohorts include patients with severe aortic stenosis, mitral regurgitation, acute pulmonary embolism or serious cardiac arrhythmias.
An immediate priority in the work-up of a case of suspected AHF is to identify patients with either cardiogenic shock (CS) and/or respiratory failure. These are among the approximately 10% of patients who are critically ill and require intensive care.
Systemic blood pressure is an important guide to the classification and management of AHF. A systolic blood pressure level <90 mmHg is encountered in about 10% of patients, but the occurrence of hypotension of this degree, in conjunction with evidence of inadequate peripheral perfusion, identifies those who are candidates for inotropic therapy and, possibly, vasopressors. These patients usually correspond to the ‘wet and cold’ quadrant of the AHF clinical classification, which is associated with notably poor prognosis.14
From a pathophysiological perspective, a key aspect of HF is that it flattens the increase in cardiac output to a given afterload, giving rise to ‘forward’ failure. Use of inotropic drugs can be a valid response to this situation, but the repertoire of available agents is restricted. Indeed, it may be argued that levosimendan is one of the few inotropes for which a compelling justification of use can be provided, and in some circumstances it is perhaps the only one.
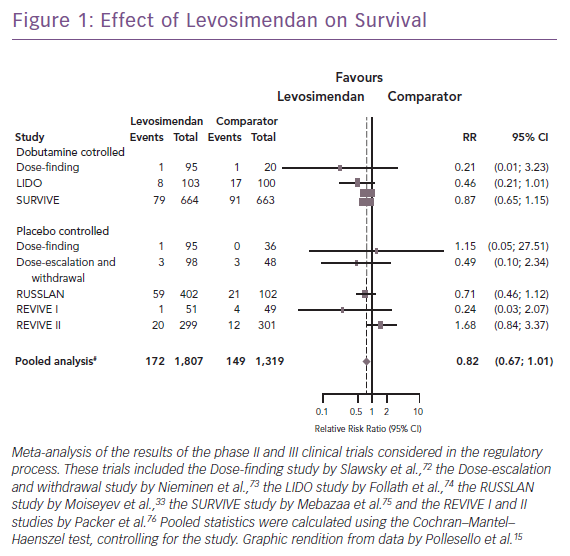
Clinical trials and meta-analyses conducted over the past quarter of a century have repeatedly indicated that conventional adrenergic inotropes and phosphodiesterase (PDE)-3 inhibitors increase cellular energy consumption and are sometimes associated with increased mortality, arrhythmias, or other safety concerns. By contrast, levosimendan does not cause an increase in cellular oxygen demand or calcium content, thus having a more favourable safety profile, as seen in an overview of the long-term mortality outcome of the regulatory clinical trials (Figure 1). Levosimendan, which has been in clinical use for more than 20 years and has been evaluated in controlled clinical trials involving >3,000 HF patients, represents an established inotropic therapy in AHF.15
Of course, these remarks should not be regarded as carte blanche for the use of levosimendan or any other specific inotrope. Indeed, it may be argued on the basis of various sources of clinical evidence that inotropes are, in general, overused in AHF, whereas vasodilators are possibly underused.16–19 Appreciation of causative pathophysiology is central to correcting this situation. AHF is a phenotype suitable for treatment with vasodilators, as the product of vasoconstriction with increase in venous return, increased left ventricular pressure and fluid redistribution leading to pulmonary congestion. Inotrope therapy is properly confined to AHF arising from a low cardiac output condition. A few observations highlight the need to improve the identification of patients who really need inotropic support (and perhaps the selection of the most appropriate inotrope for any particular case).13
However, within that qualifying population, inodilators, such as levosimendan, should be the therapy of preference for patients already receiving beta-blockers, those with AHF of ischaemic aetiology and those experiencing cardiorenal syndrome. Aspects of renal function in AHF and AdHF are considered later in this article.
Levosimendan in Acute Heart Failure or Cardiogenic Shock Arising from Acute Coronary Syndromes
AHF in the context of acute coronary syndromes (ACS) is an urgent situation that requires early identification and treatment, not least because AHF can deteriorate into CS. Risk factors for the emergence of AHF in ACS include advanced age, previous MI or chronic HF, diabetes, hypertension and female sex.
More than 40% of cases of AHF were encountered with episodes of ACS in the EuroHeart Failure Survey II, and the combination of ACS with AHF has been associated with very poor survival prospects.18 The Finnish Acute Heart Failure (FINN-AKVA) study documented an almost twofold higher 30-day mortality in AHF patients with ACS than in non-ACS cases (13% versus 8%; p=0.03).20 ACS–AHF was also associated with prolonged hospitalisation and with more costly treatment in the intensive care unit. Similar adverse findings for the interplay between ACS and AHF have been recorded in the CardShock study and other investigations.21
As evidenced by the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry, both the incidence of AHF as a complication of ACS and the mortality associated with ACS–AHF have decreased in recent years: between 1996 and 2008, the incidence of AHF as a sequel to ACS declined from 46% to 28% (p<0.001).22 This downward trend has been particularly marked in patients with ST-segment elevation MI and is very likely attributable to a more frequent use of primary percutaneous coronary intervention (PCI), which assures early reperfusion and salvage of jeopardised myocardium, thereby averting the emergence of AHF.23 The use of more high-sensitivity troponin testing to enhance detection of minor evolving ischaemia may also have contributed to this trend.
The results of the Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial make a strong case for favouring a culprit-lesion-only strategy in most patients when performing PCI for ACS–AHF.24 The short-term risks associated with longer procedure times, more complex interventions and higher doses of contrast agents seem to outweigh any potential benefits of a multivessel approach.
There is extensive polypharmacy in ACS–AHF, with widespread use of inotropes, vasopressors and other classes of drugs, but many of these practices are empirical and pragmatic rather than evidence based.20 Formal structured research into the relative merits of different drug therapies in ACS–AHF is lacking and there is insufficient reliable information regarding the comparative efficacy of different agents.25,26
Some broad principles of therapy may nevertheless be identified. Several of these apply with special force to the management of CS, the emergence of which is identified in the 2016 ESC guidelines as warranting consideration of inotrope use.13 The percentage of ACS episodes that progress to CS is relatively low (≤10%), but short-term (in-hospital) mortality in CS is exceptionally high (40% in CardShock, higher in other reports) and CS is the leading cause of death in patients with acute MI.21,23,27
The management of CS includes haemodynamic support with inotropes and vasopressors to increase cardiac output and blood pressure in order to restore tissue perfusion. Inotropes as a broad class are endorsed to support the circulation of patients who are demonstrably hypotensive and/or hypoperfused despite adequate filling pressures. This circumscribed indication reflects concerns that conventional adrenergic inotropes (and PDE-3 inhibitors) increase cellular energy demands and oxygen consumption in a situation of ischaemic compromise and may exert undesirable tachycardic or pro-arrhythmic effects. Levosimendan, by virtue of its calcium-sensitising action, does not exert untoward effects of this kind to the same degree and, moreover, exhibits anti-stunning and condition effects that may be relevant and advantageous in states of ischaemia.28–32 The survival benefit of levosimendan in the Randomized Study on Safety and Effectiveness of Levosimendan in Patients with Left Ventricular Failure after an Acute Myocardial Infarct (RUSSLAN) trial supports those considerations, as do the findings of a meta-analysis of six studies (n=1,065), which documented improvements in various indices of haemodynamic function in ACS patients treated with IV levosimendan, with no adverse effect on mortality in AHF–CS patients and a strong signal for a survival benefit in AHF–ACS patients (RR 0.74, 95% CI [0.58–0.93]; p=0.01).33,34
The Survival of Patients with Acute Heart Failure in Need of Intravenous Inotropic Support (SURVIVE) trial compared levosimendan and dobutamine in AHF.25 For the subset of patients who had acute MI as a cause of AHF, mortality in both treatment groups was two to three times that in the non-ischaemic subset and 31-day mortality was 4% lower in the levosimendan ACS–AHF group (28% versus 32%), a notable, although not statistically significant, survival gain.
It has been proposed that levosimendan may be considered in four clinical AHF–CS scenarios based on a patient’s haemodynamic status and Killip classification.25 In the lower Killip classes, and in patients with relatively well-sustained blood pressure (systolic >110 mmHg), levosimendan may be used as monotherapy to enhance urinary output if the response to diuretics is inadequate. In the more advanced stages with pulmonary oedema or frank CS, levosimendan may be combined with a vasopressor such as noradrenaline to augment cardiac output and raise blood pressure.
When vasopressors are used to support blood pressure there should be a strong presumption for noradrenaline over adrenaline, based on data from a head-to-head controlled comparison in CS and findings from CardShock.35,36
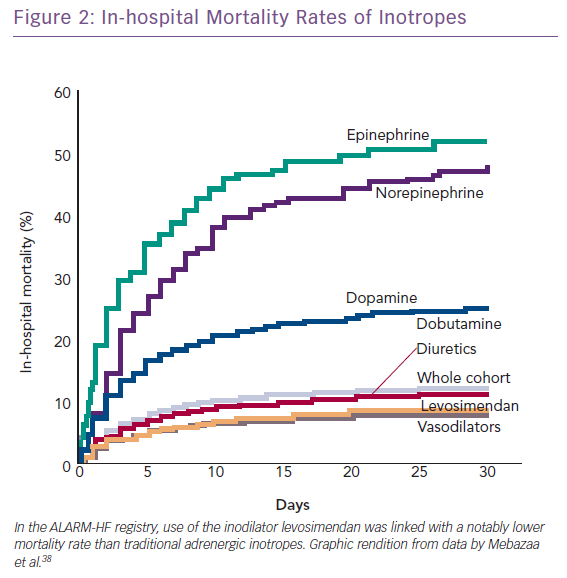
The combination of vasopressors plus inodilators may offer better short-term prognosis than vasopressor therapy alone (HR 0.66, 95% CI [0.55–0.80]). This proposition is based on a pooled analysis from three observational studies and requires confirmation in a suitably powered randomised controlled trial.37 The Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) registry, which contributed data to this analysis, indicated within a single dataset of reasonable size (n=4,953) that inodilatation as delivered by levosimendan was associated with substantially better survival than inopressors or adrenergic inotropes (Figure 2).38 This identifies, subject to confirmation, a niche for levosimendan, which may be used in combination with noradrenaline as an alternative to dobutamine. Of note in this context, the blood pressure-lowering effect of levosimendan does not appear to require excessive increases in vasopressor dosage in CS.39 Because its inotropic effect is independent of beta-adrenoceptor stimulation, levosimendan is an appropriate haemodynamic support for ACS–AHF or CS patients on chronic beta-blocker therapy.
All inotropes and vasopressors should be used at the lowest dose and for the shortest time possible. Levosimendan should be administered at an individualised infusion rate in the range 0.05–0.2 µg/kg/min. Loading dose is to be used when in need for immediate effect, and if systolic blood pressure exceeds 100 mmHg. Ideally, the infusion rate should be closely monitored and individualised in dependency of tolerability and haemodynamic response. Hypovolaemia and/or hypokalemia must be corrected before and during treatment. The effects of a 24-hour infusion of levosimendan persist for up to 2 weeks due to the long-lasting effect of its active metabolite, but the haemodynamic effects may be longer. Thereafter, treatment may safely be repeated.
Levosimendan in Advanced Heart Failure
Patients with AdHF suffer from severe and persistent symptoms that are often intractable to recommended drug therapies; they typically have marked limitation of exercise capacity and accompanying impaired QoL and are likely to have undergone repeated hospitalisations.40 AdHF is also widely associated with progressive deterioration in the function of multiple organ systems, including the kidneys and liver. AdHF may affect up to 10% of patients with HF and this prevalence may be expected to increase in future because of growth in the HF population and improved survival among AdHF patients.
Some published studies and some preliminary observations on the physiological effects of levosimendan in AdHF provide a starting point for an appraisal of the drug’s use in this context. A series of recent studies has examined the impact of levosimendan treatment on the lungs, heart and skeletal muscle.41–43 Collectively, these studies provided evidence that single-dose levosimendan administration to AdHF patients was accompanied by:
- improved peak oxygen uptake and amelioration of ventilation efficiency;
- reduced brain natriuretic peptide (BNP);
- increased cardiac output at rest and during exercise;
- improved lung mechanics and diaphragm function;
- restoration of the normal function of alveolar capillary cells (but not of alveolar capillary gas diffusion); and
- improved oxygen delivery to the muscle and muscle oxygen utilisation.
The Heart Failure Association of the ESC reviewed its definition of AdHF in 2018.44 In our collective opinion, this revised definition provides the best available starting point for a consideration of treatment options, with the proviso that it is not a guideline and that it offers neither classes of recommendation nor formal, structured levels of evidence.
Heart transplantation (HTx) remains the definitive intervention in AdHF and delivers very good outcomes.45 However, donor shortage limits this option to a minority of patients who must be carefully selected from those who are simultaneously at high risk of dying without a transplant and who may be expected to have good prognosis after receiving a donor heart.46
For the many patients rendered ineligible for HTx by virtue of age and/or co-morbidities or by the absence of a donor heart, long-term mechanical circulatory support (MCS) with continuous flow left ventricular assist devices (LVADs) may now be a valid alternative destination therapy (DT). About half of the >2,500 LVADs implanted annually in the US are intended as DT measures. Contemporary registries report good survival with LVADs as DT (78% and 68% at 1 and 2 years, respectively between 2013 and 2016 in the International Society for Heart and Lung Transplantation Mechanically Assisted Circulatory Support [INTERMACS] registry).47
Complication rates with MCS remain tangible, but the risk of death, disabling stroke and device reoperation has been substantially reduced with the advent of newer devices.48,49
Many AdHF patients falling outside the parameters for HTx or MCS receive inotropes to stabilise their haemodynamic status and relieve symptoms. Repeated scheduled infusions of drugs, such as dobutamine or PDE-3 inhibitors, should be avoided because of concerns about malignant arrhythmias and increased mortality.50–52
In contrast, the intermittent use of levosimendan has been shown to be safe and well tolerated; neither the LEVO-Rep nor LION-HEART randomised controlled trials produced indications of increases in all-cause mortality or sudden cardiac death during four and six cycles, respectively, of levosimendan therapy.53,54 In addition, levosimendan offers persistent haemodynamic improvement thanks to a pharmacologically active metabolite with a long half-life.
A survival effect of intermittent levosimendan has not been demonstrated in a properly powered randomised controlled trial, but the results of the Pulsed Infusions of Levosimendan in Outpatients With Advanced Heart Failure (Levo-Rep) and Intermittent IV Levosimendan in Ambulatory Advanced Chronic Heart Failure Patients (LION-HEART) trials make a persuasive case for further evaluation of levosimendan in this context.53,54 The Repetitive Levosimendan Infusion for Patients With Advanced Chronic Heart Failure (LeoDOR) trial is currently recruiting patients for this purpose (NCT03437226). This multicentre randomised controlled trial is designed to explore the safety and efficacy of repetitive levosimendan infusions (seven cycles at 0.2 µg/kg/min for 6 hours every 2 weeks or five cycles at 0.1 µg/kg/min for 24 hours every 3 weeks) administered to AdHF patients following a recent HF-related hospitalisation.
As many as 80% of AHF hospitalisations are the product of acute-on-chronic deterioration in haemodynamic status; this may include cases where AHF is superimposed on AdHF.40 As was exemplified in the findings of the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) study, congestion and dyspnoea precede the emergence of AHF; more generally, haemodynamic congestion precedes symptomatic congestion, which in turn precedes hospitalisation for AHF.55 As described by Zile et al. and conceptualised by Adamson, the phase of presymptomatic congestion may precede the emergence of overt clinical symptoms by several days to weeks.56,57
The existence of this period of preclinical decline represents an opportunity for intervention that may avert unplanned hospitalisation due to haemodynamic crisis. Given that repeat hospitalisation for AHF is associated with progressively deteriorating survival prospects, identifying and exploiting this opportunity for pre-emptive treatment is clearly in the interests of patients. Observations on the feasibility of pre-symptomatic intervention to avert hospitalisation add weight to observations in the LEVO-Rep and LION-HEART trials that use of intermittent levosimendan in outpatients with AdHF was associated with marked improvement in event-free survival (LEVO-Rep) or a reduction in HF hospitalisation (LION-HEART).53,54,58,59 A recent meta-analysis of six studies of intermittent levosimendan in chronic HF has produced an estimated risk ratio of 0.40 (95% CI [0.27–0.59]; p<0.00001), with consistency of effect in all the contributing studies.60
Differential Renal Effects of Levosimendan
Kidney dysfunction is encountered in a substantial proportion of patients with AHF or AdHF.61 In this setting, it is usually secondary to impaired cardiac function, conforming to the definition of type 1 cardiorenal syndrome (CRS). Various pathophysiological mechanisms contribute to kidney damage in CRS, including hypoperfusion, renal venous congestion and neurohormonal activation.
Renal dysfunction has repeatedly been shown to be one of the most adverse prognostic indicators for patients with HF and to be linked with prolonged hospitalisation.62–65 Therefore, pharmacological and non-pharmacological interventions for AHF or AdHF need to be shaped by the ambition to preserve or rectify renal perfusion, the deterioration of which underlies the emergence of kidney dysfunction.
The use of inodilators or inotropes to avert or correct CRS may be particularly apt in patients with low blood pressure or hypoperfusion and the specific effects of levosimendan on renal vasculature and haemodynamics highlight its potential in these cases.66–68 Those effects include selective vasodilation of the renal glomerular afferent arterioles, thereby enhancing renal filtration directly as well as via its effect on cardiac output.
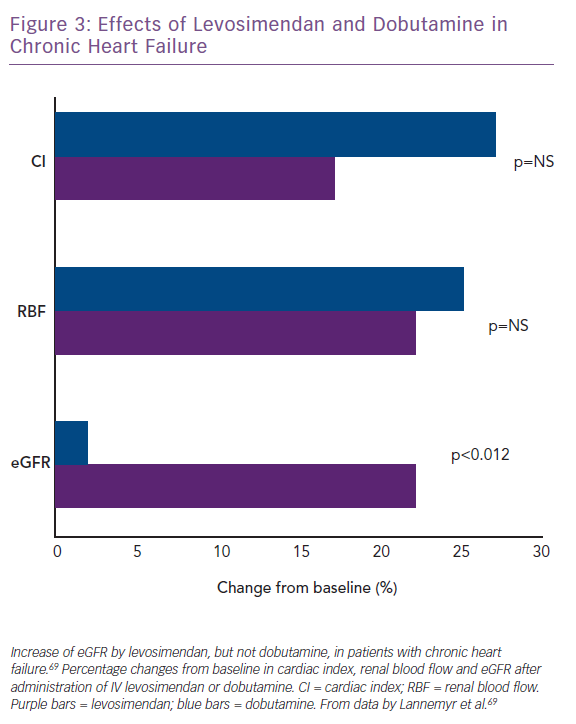
Lannemyr et al. recently reported that both levosimendan (loading dose of 12 µg/kg for 10 minutes, then infusion at 0.1 µg/kg/min for 65 minutes; n=16) and dobutamine (continuous infusion started at 5.0 µg/kg/min for 10 minutes, then 7.5 µg/kg/min for 65 minutes; n=16) improve systemic haemodynamics and renal blood flow to a similar extent in patients with chronic HF (mean baseline left ventricular ejection fraction 27%) and impaired renal function (mean eGFR <80 ml/min/1.73 m2).69 However, only levosimendan increased eGFR (Figure 3), supporting the proposition that levosimendan causes selective vasodilation of afferent renal arterioles whereas dobutamine dilates both afferent and efferent vessels. These data indicate that the similarity of effect on systemic haemodynamic indices may not translate into correspondingly favourable effects on renal perfusion and signal that levosimendan may be a preferred inotropic agent for the management of CRS in the setting of low-output AHF or AdHF.
Case studies reviewed at Heart Failure 2019 illustrate that levosimendan may also be appropriate as part of a bridge to transplant strategy for preserving renal function in patients with AdHF and restrictive cardiomyopathy. A series of 35 repeat courses of levosimendan therapy delivered over 20 months was associated with large and sustained improvements in a series of indicators of renal function, including creatinine, N-terminal pro-BNP and the need for oral potassium supplementation. This intervention brought creatinine levels, the most responsive and most quickly reacting indicator of haemodynamic effects on kidney function in CRS, into the normal range for six consecutive months before further clinical deterioration necessitated HTx. These experiences are consistent with an earlier report of long-term improvement in renal function in a prospective study of 40 patients with AdHF treated with levosimendan while awaiting HTx.70
Conclusion
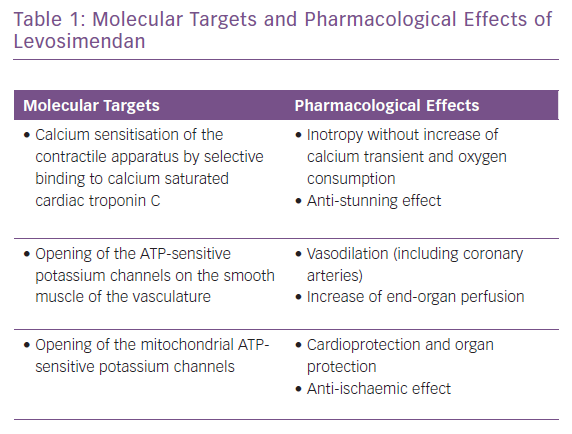
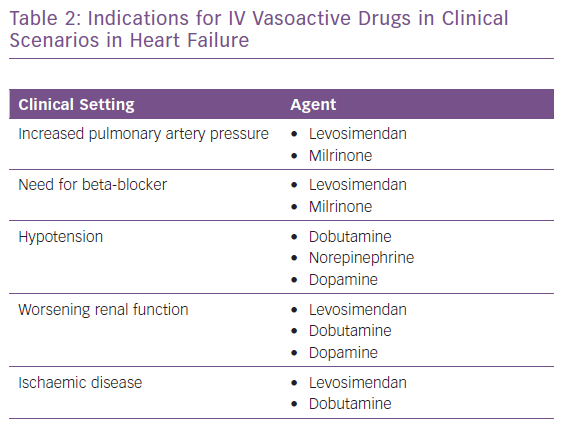
The appropriate, effective and successful use of IV vasoactive drugs in AHF and AdHF is founded on accurate assessment of the aetiology of decompensation and the broader patient profile. Where congestion or hypertension predominate, and patients present with either fluid accumulation or fluid redistribution, the management emphasis should favour vasodilators and diuretics to unload the heart and mobilise fluid. Inotropes and/or vasopressors are indicated for ‘wet and cold’ patients who exhibit inadequate peripheral perfusion despite adequate filling status. These patients usually present with low blood pressure (systolic <90 mmHg), but it should be kept in mind that hypoperfusion is not synonymous with hypotension; hypoperfusion may not always be followed by significant hypotension, as in the presence of sympathetic overactivation causing peripheral vasoconstriction. Levosimendan is an inodilator with a unique pharmacology (Table 1), and may be appropriate for similar patients with higher blood pressure if they are refractory to vasodilator and diuretic therapy.71 A series of clinical scenarios warranting the use of inotropes/inodilators and/or vasopressors is shown in Table 2 and illustrates the wide-ranging utility of levosimendan as an intervention in these situations.