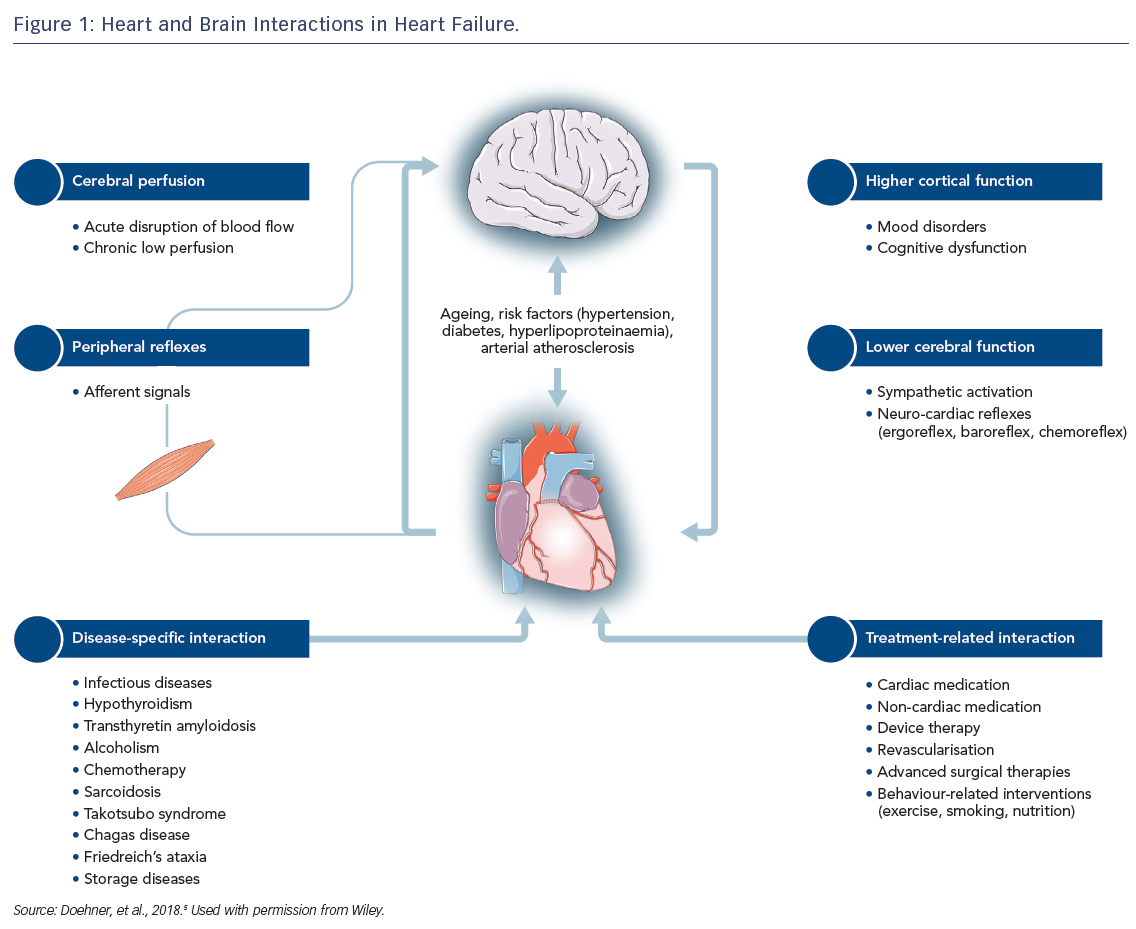
Heart failure (HF) is a complex clinical syndrome with more than 15 million diagnosed cases worldwide.1,2 Characterised by structural or functional impairment of ventricular filling or ejection fraction (EF)3, HF is frequently accompanied by multiple comorbidities. Brain disorders, including stroke, mental disturbances and cognitive impairment are distinct from the comorbidities traditionally related to HF and require specific management. Both organs are linked by multiple feedback signals, and the discovery of bi-directional interactions of failing heart and neuronal signals has led to the concept of the cardio-cerebral syndrome in HF.4 This article provides a condensed version of the recently published position paper from the Study Group on Heart and Brain Interaction of the Heart Failure Association and details several pathophysiological and functional aspects of the heart–brain interactions in HF (Figure 1).5
Stroke and Cerebral Perfusion in Patients with Heart Failure
Stroke is one of the leading causes of mortality and disability in adult life and had a global incidence rate of more than 10 million in 2013.6,7 Patients with HF have an increased risk for stroke,8 and it contributes to morbidity and mortality in this patient group.9 In the population-based Framingham Heart Study, the relative risk of stroke in people with HF was four-fold higher for men and three-fold higher for women compared with patients without HF.8 The prevalence of stroke did not differ between patients with HF with preserved ejection fraction (HEpEF) and those with HF with restricted ejection fraction (HFrEF) and ranged between 2.4 and 5.8 % for HFrEF and between 3.8 and 7.4 % for HFpEF in clinical trials.10–12 This overall reduction in risk may be related to the treatment of the disease and initiation of stroke prevention measures.
Several risk factors for stroke in patients with HF have been established. A hypercoagulable state, with activated coagulation and disturbances in proteolytic systems,13 reduced blood flow, inflammation and endothelial dysfunction, has been implicated in the development of systemic cardioembolic events in HF including stroke. Further factors such as low flow patterns due to an enlarged left atrium14 or reduced contractility of the left ventricle (LV) with apical akinesia or aneurysm represent additional risk factors of intracardiac thrombosis and, consequently, embolic stroke in patients with HF.15,16 Incidence reported in 10 small-scale observational case-control studies show wide variations in the incidence of thrombo-embolic events with a range of 1.4–12.5 % in HF patients including those with atrial fibrillation (AFib) and those receiving oral anticoagulation therapy.17
Further risk factors such as small vessel disease and large artery atherosclerosis are common in people with ischaemic HF.18 In patients with carotid artery stenosis, reduction of perfusion pressure due to systolic HF may result in a greater volume of ischaemic lesion.19 In addition, a cerebral lesion may remain clinically undetected as a so-called “silent” infarction. The prevalence of silent cerebral infarctions is comparably high in HF cohorts ranging between 27 and 63 %,20,21 which is higher than in age-matched subjects without HF.22 Silent cerebral infarctions and other structural brain damages, such as increased white matter hyperintensities24 or grey matter loss,24 are frequently found in imaging tests for HF patients with cognitive dysfunction and dementia.25
While the benefit of antithrombotic therapy in the context of AFib is clearly established regardless of the presence of HF, there is no adequate antithrombotic therapy for stroke prevention in HF patients with maintained sinus rhythm. The Warfarin versus Aspirin in Patients with Reduced Cardiac Ejection Fraction (WARCEF) trial revealed no overall difference between warfarin and aspirin in preventing ischaemic stroke in HF patients with a mean reduced left ventricular ejection fraction of 24.7 % (±7.5) and sinus rhythm.26 A reduced risk of ischaemic stroke after warfarin was equalised by the increased risk of major bleeding. A borderline significant benefit of warfarin on the primary outcome (ischaemic stroke, haemorrhagic stroke or death from any cause) was observed only after 4 years. The analysis of two smaller randomised controlled trials, the Heart Failure Long-term Antithrombotic Study (HELAS)27 and Warfarin/Aspirin Study of Heart Failure (WASH),28 demonstrated no benefit for patients with HF having antithrombotic therapy compared with placebo regarding vascular events and mortality.29 Accordingly, a position paper from a European Society of Cardiology working group does not support the routine use of warfarin in patients with HF and sustained sinus rhythm.17 It should be noted, however, that the risk–benefit ratio might be significantly improved with the introduction of novel anticoagulant (NOAC) therapies, and the results from the WARCEF trial may be outdated.
While stroke represents an acute case of low cerebral perfusion, chronic low cerebral perfusion may manifest in a series of structural cerebral alterations of grey and white matter damage in HF patients.30,31 Vascular auto-regulation of the cerebral vasculature (the Bayliss effect) enables maintenance of normal perfusion even with severely elevated blood pressure and it protects the brain against blood pressure peaks. Regional hypoperfusion may occur at low perfusion pressures, and chronic low perfusion may account for metabolic impairment, structural decrease and eventual functional decline of brain areas involved in autonomic, neuropsychological and cognitive control.32 Regional vascular recruitment is modulated by functional activity and local oxygen demands and is locally controlled by a range of factors addressed by the ‘neurovascular unit’, a heterogeneous structure composed of different cell types including astrocytes, pericytes, endothelial cells of the blood brain barrier, microglia and neurons.33
Regional hypoperfusion has been observed in multiple brain areas in people with HF, largely lateralised towards the right side in the occipital, temporal, frontal and parietal regions.34 Bilateral areas of reduced blood flow were observed in the prefrontal cortex, frontal white matter, anterior corpus callosum, thalamus, hippocampus, amygdala and occipital cortex. The decreased regional perfusion may contribute to the autonomic, mood and cognitive regulatory deficits observed in HF. Further, impaired perfusion of multiple brain areas involved in the control of vision, language and speech have been observed that could explain the respective deficits in HF patients.34
Higher Cortical Function in Patients with Heart Failure
There are two patterns of cognitive problems in HF that are recognised in clinical practice: a chronic, progressive decline in cognitive ability and an acute change in cognition associated with decompensated HF. Cognitive decline in executive function, attention, episodic memory, language, psychomotor speed and visuospatial ability is typical for patients with HF, with differences between HFrEF and HFpEF.35,36 Accelerated cognitive decline may result from chronic hypoperfusion over the long-term course of HF. The prevalence of early-onset cognitive impairment ranging from 25–74 % has been observed in patients with HF, and is associated with early death, loss of functional independence, worse adherence to therapy and decreased quality of life.37,38,39,40
Delirium, a common sequela of decompensated HF, is associated with prolonged hospital stays and increased mortality.41 Despite its high rate and severe clinical impact, the relationship between acute delirium and HF has not been studied in detail. Cognitive decline is also observed in patients with acute decompensating HF (ADHF), and one study has shown that cognitive performance with respect to memory, perceptual speed, and executive control was affected more severely in 20 patients with ADHF compared with 20 patients with stable chronic HF.42 Another clinical trial demonstrated that 80 % of 744 patients with ADHF had cognitive impairment in at least one of the cognitive domains, such as processing speed, memory and executive function.43 A correlation between cognition and markers of haemodynamic performance (left ventricular EF and N-terminal prohormone of brain natriuretic peptide) as well as inflammation (C-reactive protein) suggests that hypotensive blood pressure and haemodynamic failure plays a role in cognitive impairment.42
Mood and anxiety disorders in HF have been investigated in several clinical trials, and depression in patients with HF has become a major focus of research in recent years. Clinical studies have observed that depression is associated with poor quality of life, lower treatment adherence, greater morbidity and mortality, increased hospitalisation and higher healthcare costs for patients with HF.44,45,46 The aggregated prevalence of depression in patients with chronic HF is 21.5 %47 compared with 2.3–4.7 % in the general population.48,49 Elevated prevalence has been linked to more severe functional class and differences were observed between patients with New York Heart Association class 2 and 3 HF. However, data reporting the prevalence of depression are variable because of the use of different assessment methods, the heterogeneity of cohorts and the wide range of depression symptoms.
Anxiety is another frequently encountered disorder in HF patients with a prevalence ranging from 9–53 %.50 Anxiety in people with HF is related to older age, low level of education, poor socioeconomic status, previous psychiatric disease, decreased quality of life, multiple hospitalisations, increased natriuretic peptide levels and impaired functional capacity.51 Depression and anxiety appeared as independent predictors of all-cause mortality in a meta-analysis of 31 studies with 1–3 years’ follow-up.50 Treatment of depression with selective serotonin reuptake inhibitors for people with HF has not been successful and the results of two major randomised controlled trials (SADHART52 and MOOD-HF53) did not show significant improvement in depression scores and HF outcomes. However, in observational small-scale studies, effective management of HF-related physical symptoms improved anxiety and depression scores significantly.54 Disease management programmes and aerobic exercise seem to be as effective as drug therapy,55 and repeated visits from nurses and routine contact calls from healthcare staff to give education and care support were found to reduce hospital readmissions and increase quality of life.56
Peripheral Reflexes and Brain Stem Function
The impact of the central nervous system on vegetative control of the cardiovascular system is not fully understood. Cardiovascular signals from chemo-, baro- and ergoreceptors trigger afferent signals to the autonomic nervous system (ANS) control centres that provide efferent sympathetic and parasympathetic signals to form baro-, metabo- or chemoreflex circuits. Imbalanced neuroendocrine activation and control of the myocardium and circulation is fundamental in HF pathophysiology and is a driving force of disease progression and high mortality. Peripheral chemoreceptor hypersensitivity characterised by increased sympathetic drive and hyperventilation is predictive of poor outcome in patients with chronic HF.57 During exercise, the contribution of the muscle ergoreceptors to autonomic, hemodynamic, and respiratory responses among patients with HF has been shown to be enhanced compared with control subjects,58 leading to hyperventilation and intolerance of exercise.59 In addition, reduced values of the autonomic markers (heart rate variability and baroreflex sensitivity) were associated with increased mortality after myocardial infarction.60
The ANS is an important target for research into HF therapies.61 Impaired signals between the heart, the cortex and brain stem caused by low perfusion might lead to alterations of the ANS with increased sympathetic tonus, parasympathetic withdrawal and impaired neurocardiac reflexes.30,32,62 Indeed, regional cerebral blood flow to the frontal cortex fails to rise in HF patients during exercise when compared with healthy controls.63 Experimental and clinical studies have also shown an association between stroke and increased levels of catecholamines and/or abnormal autonomic control of heart rate (heart rate variability) and arterial baroreflex sensitivity.64,65 The activation of the sympathetic nervous system, especially after injury involving the insular cortex, promotes the development of AFib, ventricular arrhythmias and abnormalities in QT interval.66,67 Alterations in blood pressure, heart rate and breathing control that derive from a reduction in baroreflex sensitivity and a concomitant increase in peripheral and central chemosensitivity, lead to a pattern of reflex instability. This pattern, known as Cheyne-Stokes respiration, is observed in advanced HF and manifests as central sleep apnoea. The Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure (SERVE-HF) trial found an increased mortality rate in participants which highlights the importance of this mechanism when central sleep apnoea in patients with advanced HFrEF was treated with adaptive servo-ventilation.68 The mechanism may be related to the adverse hemodynamic effects of positive airway pressure in HFrEF patients with low EF although the exact mechanism remains uncertain.
Treatment-related Interactions
Treatment-related interactions within the heart–brain axis can be categorised as medical, interventional or device-related. High prevalence of comorbidities in patients with HF accompanied by polypharmacy and age-related pathophysiological changes may affect the efficacy of guideline therapies. Thus, in older patients with HF, the side-effects of lowering blood pressure and the subsequent cerebral hypoperfusion might result in cognitive decline, falls and depression.69,70 However, hypertension treatment has been shown to reduce the risk of death and admission to hospital in a meta-analysis investigating more than 13,000 patients with HFrEF in sinus rhythm.71
In patients receiving device therapy, an increase in symptoms of depression and anxiety during the initial weeks after implantation have been shown.72,73 These symptoms fade, especially in patients who have a favourable response to the therapy, such as cardiac resynchonisation, and cognitive performance improves. Nevertheless, receiving a shock from an implantable cardioverter-defibrillator (ICD) can lead to emotional dysfunction, anxiety and depression during the following month.72,73 Almost 20 % of patients with an ICD suffer post-traumatic stress disorder due to a history of cardiac arrest, device implantation and ICD shock, and cognitive behavioural therapy can potentially improve outcomes.73
The beneficial effect of exercise on functional status and outcome in patients with HF has been shown in several clinical trials. A wide range of mechanisms, including indirect effects via cerebral signals such as improved sympatho-vagal balance and attenuated activation of ergo- and metaboreflexes, enhancing cerebral haemodynamics, or even cortical, anti-depressive effects of exercise might contribute to physical and functional improvement in patients with HF.74–76
Conclusion
HF is a complex clinical syndrome that involves all organs and systems of the body and it is associated with multiple comorbidities. Bi-directional interactions between failing myocardium and brain dysfunction contribute to the symptoms that patients with HF present with and they account for comorbidities such as stroke, impaired ANS functions, sleep apnoea, cognitive impairment or depression. Neuro-cardiac feedback signals significantly promote disease progression and cause a poor prognosis in patients with HF. A better understanding of interactions within the heart–brain axis is needed to improve management and prognosis of HF patients.