In 2016, the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)1 introduced heart failure (HF) with mid-range ejection fraction (HFmrEF) as a distinct phenotype. This distinction was expected to stimulate research on the underlying characteristics, pathophysiology and treatment of patients with HFmrEF. Indeed, in the following 2 years, the number of studies devoted to HFmrEF grew rapidly. Given that in terms of left ventricular ejection fraction (LVEF) HFmrEF (LVEF 40–49 %) occupies an intermediate position between HF with reduced ejection fraction (HFrEF) (LVEF <40 %) and HF with preserved ejection fraction (HFpEF) (LVEF >50 %), the key question is whether patients with HFmrEF represent a distinct pathophysiological entity or a transitional phenotype between HFrEF and HFpEF. The search for an answer to this question continues and will determine the effectiveness of strategies for the management of patients with HFmrEF.
This article provides a narrative review of findings from recent observational studies, sub-analyses of clinical trials and analyses of data from large registries that focused on patients with HFmrEF. This review aims to discuss the current state of evidence regarding the essence of HFmrEF and management of patients with HFmrEF.
Terminology Related to Heart Failure with id-range Ejection Fraction
In 2014, the term HFmrEF was proposed for the first time by Lam and Solomon2 to describe patients with HF and LVEF in the range of 40–49 %, who had been commonly excluded from clinical trials. Since then, HFmrEF has often been called the ‘middle child’ in the HF family, implying the lack of attention to this phenotype in comparison with its ‘siblings’ HfrEF and HFpEF.2 In the 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic HF,1 the term HFmrEF replaced the term ‘grey area’ that had been previously used to refer to HF patients with LVEF of 35–50 % and mild systolic dysfunction. According to these ESC Guidelines, a positive diagnosis of HFmrEF requires the following conditions to be fulfilled: (i) symptoms and/or signs of HF; (ii) LVEF of 40–49 %; (iii) elevated natriuretic peptides (B-type natriuretic peptide – BNP ≥35 pg/ml or N-terminal pro-B type natriuretic peptide – NT-proBNP ≥125 pg/ml); and (iv) a relevant structural heart disease (left ventricle hypertrophy with left ventricular mass index ≥115 g/m2 for men and ≥95 g/m2 for women) or left atrial enlargement (>34 ml/m2) or diastolic dysfunction (the ratio of mitral peak velocity of early filling – E to early diastolic mitral annular velocity – e’, E/e’ ratio ≥13 and a mean e’ septal and lateral wall <9 cm/sec).1
Similarly, the 2013 American College of Cardiology/American Heart Association (ACC/AHA) Guidelines3 described patients with LVEF in the range of 40–50 % as an intermediate group, pointing to its certain similarities to the group of patients with HFrEF. Besides that, the 2013 ACC/AHA Guidelines3 acknowledged the existence of borderline HFpEF (HFbEF) (LVEF 41–49 %) and improved HFpEF (LVEF >40 %). Such HF patients are classified as HFpEF patients; only in the first case they fall into a borderline or intermediate group, and in the second case they previously had HFrEF with a later improvement or recovery of LVEF.
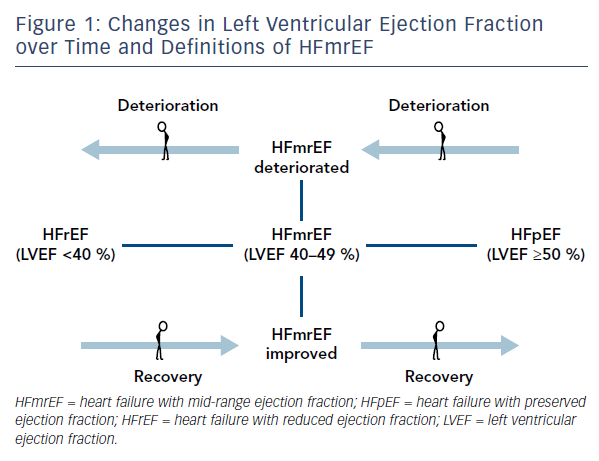
The recognition of the fact that LVEF often changes over time4,5 determined the appearance of such clarifying definitions as HFmrEF improved (previously HFrEF with LVEF <40 %), HFmrEF deteriorated (previously HFpEF with LVEF >50 %) and HFmrEF unchanged (previously HFmrEF with LVEF 40–50 %)6 (Figure 1). For such definitions, more than one measurement of ejection fraction is required. Nonetheless, in 2017 Lam and Solomon6 recognised that research should focus on finding prognostic differences and responses to therapeutic intervention across the spectrum of EF rather than terminology.
Pathophysiology of Heart Failure with Mid-range Ejection Fraction
Limited evidence is currently available with respect to pathophysiological mechanisms of HFmrEF.7 A measurement of 37 biomarkers from different pathophysiological domains (myocardial stretch, inflammation, angiogenesis, oxidative stress, haematopoiesis) showed that biomarker profiles in patients with acute HFrEF were mainly related to cardiac stretch while biomarker profiles in patients with HFpEF were mainly related to inflammation.8 However, patients with HFmrEF demonstrated an intermediate biomarker profile with biomarker interactions between both cardiac stretch and inflammation markers.8 Further studies on the pathophysiology of HFmrEF are required.
Prevalence and Clinical Characteristics of Heart Failure with Mid-range Ejection Fraction
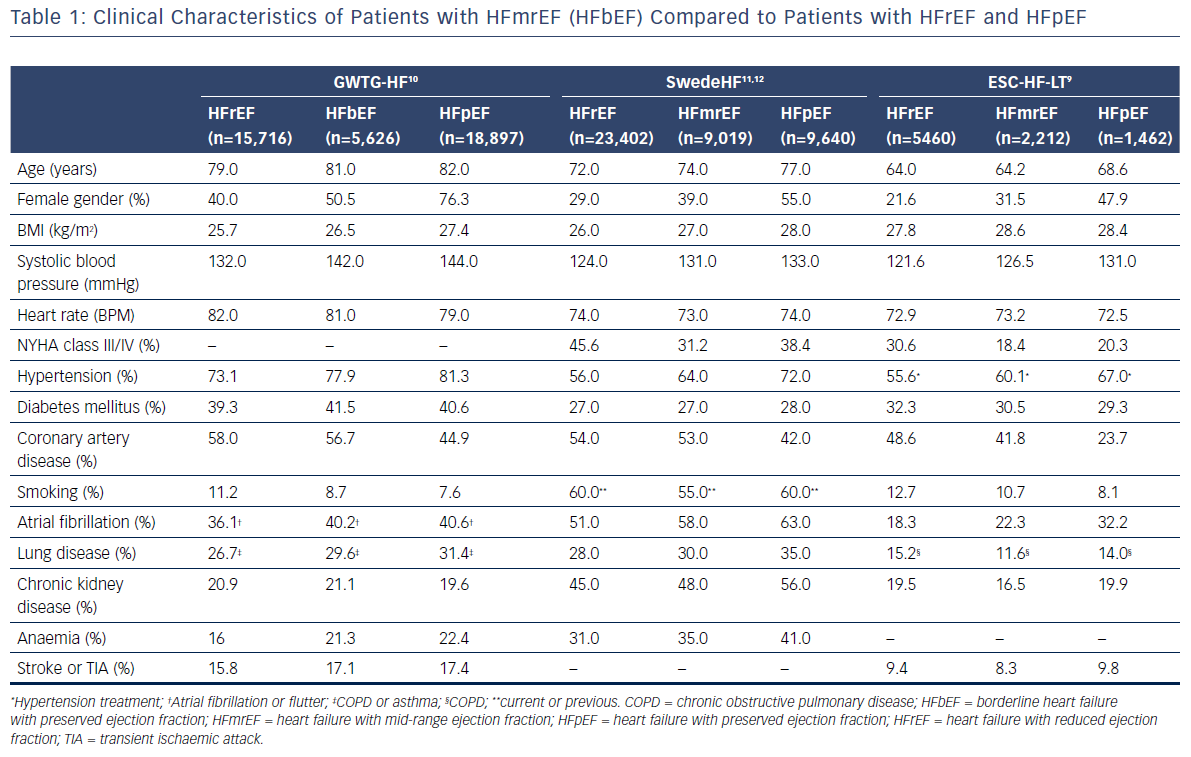
Based on the results of recently-published clinical studies and analyses of data from registries, patients with HFmrEF may constitute up to one-quarter of all patients with HF.7,9–15 Clinical characteristics of patients with HFmrEF compared with those of patients with HFrEF and HFpEF, which were identified through analyses of data from three large registries,9–12 are presented in Table 1. Despite some similarities in clinical presentation, burden of comorbidities, and quality of life between HFrEF, HFmrEF, and HFpEF, in general, patients with HFmrEF were often characterised as a population of patients with intermediate characteristics between HFrEF and HFpEF. Nevertheless, patients with HFmrEF were usually younger and more likely to be male compared with those with HFpEF. In terms of these characteristics, the HFmrEF group resembles the HFrEF group but, most importantly, HFmrEF is closer to HFrEF with regard to both a higher prevalence of coronary artery disease (CAD) and a greater risk of new cardiac ischaemic heart disease (IHD) events.13 Besides, previous myocardial infarction and revascularisation procedures were more common both in patients with HFmrEF and HFrEF, than in patients with HFpEF.16 However, patients with HFmrEF were more likely to have hypertension and diabetes than those with HFrEF. Moreover, patients with HFmrEF showed a higher prevalence of atrial fibrillation and left ventricular hypertrophy but a lower prevalence of left ventricular and atrial dilation compared to patients with HFrEF.9 It should be noted that HFmrEF patients with atrial fibrillation compared with sinus rhythm were older, had a higher prevalence of hypertension, transient ischaemic attacks or stroke, a longer duration of HF and more severe HF but a lower prevalence of IHD.17 Analyses of the most common precipitating factors for hospitalisation among HF patients revealed high occurrence of respiratory infections, arrhythmias, myocardial ischaemia and medication noncompliance, regardless of the baseline LVEF.14,15 However, the prevalence of these factors as precipitants for the acute episode among patients with HFmrEF was intermediate compared with HFrEF and HFpEF.
Overall, further studies analysing patients’ demographic characteristics, as well as aetiology and clinical presentation of the disease, may improve risk stratification and management of patients with HFmrEF.
Predictors and Prognosis of Heart Failure with Mid-range Ejection Fraction
The analysis of pooled data from four community-based longitudinal cohorts identified 200 cases of HFmrEF (10 % of all new HF cases) among 28,820 participants, who were followed for a median of 12 years.18 Among clinical predictors of HFmrEF were such factors as older age, male sex, higher systolic blood pressure, diabetes mellitus and previous myocardial infarction (p<0.01 for all).18 Natriuretic peptides, cystatin-C and high-sensitivity troponin were identified as biomarkers predicting HFmrEF (p<0.01 for all).18 Interestingly, while natriuretic peptides had higher association with the incidence of HFrEF than of HFmrEF, they did not differ in their association with the incidence of HFmrEF compared with HFpEF. The rate of all-cause mortality after the onset of HFmrEF was higher than after the HFpEF (50 and 39 events per 1,000 person-years, respectively, p=0.02), but did not differ from the rate of all-cause mortality of HFrEF (50 and 46 events per 1,000 person-years, respectively, p=0.78).18
Several recently-published analyses of data from clinical registries compared the prognoses of HFrEF, HFmrEF and HFpEF, the results of which were divergent.9,11,19–23
In the Acute Heart Failure Global Registry of Standard Treatment (ALARM-HF), 4,953 patients hospitalised with the diagnosis of HF were included.19 Of those, 25 % of the patients who had a documented LVEF (n=3,257) had a diagnosis of HFmrEF. Patients with HFmrEF were more frequently hospitalised due to acute coronary syndrome (38.6 %, p<0.01) or infection (17 %, p<0.01) compared with patients with HFrEF and HFpEF. The most common presentations of HFmrEF were acute pulmonary oedema, acute de novo HF or atrial fibrillation/flutter on admission. Hazard of all-cause in-hospital mortality or 30-day mortality in HFmrEF was statistically significantly lower than in HFrEF (hazard ratio [HR]=0.64, p=0.03) but similar to HFpEF (HR=1.03, p=0.92).
The Get With The Guidelines®-HF (GWTG-HF) Registry20 included 39,982 patients from the US hospitalised with HF; of those, 8.2 % had HFbEF. The 5-year mortality rate was similar in patients with HFrEF, HFbEF and HFpEF (75.3 %, 75.7 % and 75.6 %, respectively). Patients with HFbEF had a slightly higher re-admission rate than those with HFpEF (85.7 % and 84.0 %, respectively, adjusted HR=1.05, p=0.03).
In the RICA Registry,21 2,753 patients admitted with HF to Spanish internal medicine units were included; of those, 10.2 % had HFmrEF. Patients with HFrEF had statistically significantly higher 1-year mortality compared with patients with HFmrEF and HFpEF (28 % versus 20 % and 22 %, respectively, p<0.01). However, there was no difference between the three groups in 30-day and 1-year re-admission rates.
In the REDINSCOR II Registry,22 16 % of patients admitted to Spanish cardiology services with decompensated or de novo HF (n=1,420) had HFmrEF. Over a 1-year prospective follow-up, HRrEF, HFmrEF and HFpEF groups showed no statistically significant differences in all-cause mortality, cause of death or HF re-admission. In all three groups, the most frequent cause of death was refractory HF, followed by death due to non-cardiovascular causes.
The ESC Heart Failure Long-Term Registry9 collected 1-year follow-up data in 9,134 ambulatory HF patients; of those, 24.2 % had HFmrEF. There was no statistically significant difference in 1-year all-cause mortality among patients with HFrEF and HFpEF (8.8 % versus 6.3 %, p<0.01). Patients with HFmrEF experienced an intermediate level of 1-year all-cause mortality (7.6 %), which did not differ from patients with HFrEF (p=0.07) and HFpEF (p=0.17). Non-cardiovascular mortality was higher in patients with HFmrEF and HFpEF (27.8 % and 30.7 %, respectively) compared with HFrEF (20.1 %); however, this difference was not statistically significant (p=0.06). The percentages of patients hospitalised for HF in the HFrEF group was statistically significantly higher than in the HFmrEF and HFpEF groups (14.6 %, 8.7 %, and 9.7 %, respectively, p<0.01).9
In the Swedish Heart Failure Registry,11 all-cause mortality of 42,061 hospitalised and ambulatory HF patients, 21 % of which had HFmrEF, was analysed at 30 days, 1 year and 3 years of follow-up. All-cause mortality in the overall cohort was numerically but not statistically significantly higher between patients with HFmrEF and HFpEF at all time points, while it was considerably and significantly higher in HFrEF compared with HFpEF (p<0.01 for interaction) at all time points. Nevertheless, 3-year mortality was higher in HFmrEF than in HFpEF in the presence of CAD (HR=1.11), but not in the absence of CAD (HR 1.02, p<0.01 for interaction).
Two Spanish prospective registries (Network for the Study of Heart Failure [REDINSCOR I] and the MUerte Súbita en Insuficiencia Cardíaca (MUSIC)23 included 3,446 ambulatory HF patients with a median follow-up of 41 months. Of those, 13.3 % had HFmrEF. The observed all-cause mortality was statistically significantly higher in the group of HFrEF than in HFmrEF and HFpEF, which had comparable rates (33.0 %, 27.8 %, and 28.0 %, respectively, p=0.01). The risk of cardiovascular death, HF death or sudden cardiac death did not differ between HFmrEF and HFrEF. However, patients with HFmrEF were at a higher risk of cardiovascular death (sub-hazard ratio=1.71, p=0.01) and sudden cardiac death (sub-hazard ratio=2.73, p=0.04) than patients with HFpEF.
The differences in the above-mentioned results could potentially be explained by the features of the HF patients included in these registers (acute, stable HF or their mix), as well as different periods of follow-up. Further studies are required to determine a long-term prognosis in HFmrEF patients.
Left Ventricular Ejection Fraction Transitions and Prognosis in Heart Failure Patients with Mid-range Ejection Fraction
As discussed in the introduction, LVEF in HF patients quite often shifts from one category to another, which reasonably raises the question about the transitional status of HFmrEF between HFpEF and HFrEF rather than it being a distinct phenotype of HF. Indeed, in the Swedish Heart Failure Registry,16 more than one-third of HFmrEF patients experienced worsening of EF during the follow-up, whereas approximately one-quarter improved their EF. Of note, patients with IHD in general, and new IHD events in particular, were more likely to experience worsening of EF and less likely to experience improvement in EF.16 In the Chronic Heart Failure Analysis and Registry in the Tohoku District-2 (CHART-2) study,13 HFmrEF at registration transitioned to HFpEF and HFrEF by 44 % and 16 % at 1 year, respectively. At 1 year, HFmrEF patients had mortality comparable to that of HFpEF patients, which was better than that in HFrEF patients. However, patients with HFmrEF at registration had increased mortality if they transitioned to HFrEF at 1 year. Similar data were obtained from the Washington University Heart Failure Registry.7 In this registry, the majority of patients (73 %) had HFmrEF improved (prior LVEF <40 %), 17 % had a deteriorated HFmrEF (prior LVEF >50 %), and 10 % remained stable in HFmrEF (prior LVEF 40–50 %). Herewith, patients with improved HFmrEF had significantly (p<0.01) better clinical outcomes (death, cardiac transplantation, HF hospitalisation, cardiac hospitalisation) relative to matched patients with HFrEF and to HFmrEF deteriorated patients.7 Meanwhile, clinical outcomes of the HFmrEF deteriorated subgroup of patients did not differ from the outcomes of matched HFpEF patients.
It appears that the transition of LVEF from HFrEF to HFmrEF is associated with a better prognosis than stable HFmrEF, but patients who deteriorate from HFpEF to HFmrEF have a slightly worse prognosis than patients with stable HFmrEF. Further studies are needed to confirm this observation.
Management of Heart Failure Patients with Mid-range Ejection Fraction
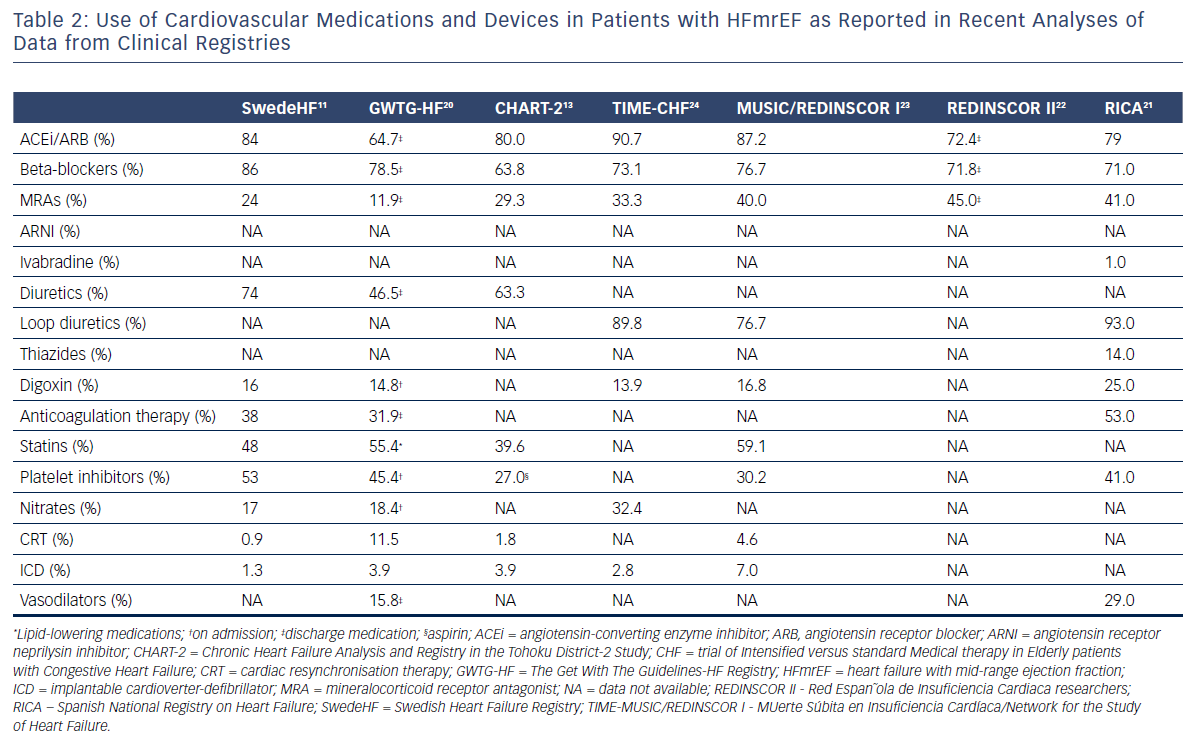
Due to the fact that some HFmrEF patients have been included in trials focused on HFpEF patients, the current ESC Guidelines on HF1 recommend therapies for HFmrEF on the basis of the evidence for HFpEF rather than that for HFrEF. Along with that, diuretics are recommended in congested patients with HFmrEF to alleviate symptoms and signs.1 Nevertheless, the rate of prescription of HF classic disease-modifying agents, especially, such as angiotensin-converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs) and beta-blockers, is quite high in patients with HFmrEF. The use of cardiovascular medications and devices in patients with HFmrEF as it was presented in the recently published analyses of data from registries is summarised in Table 2. Several analyses showed that HFmrEF patients, in contrast to patients with HFpEF, had a benefit in prognosis similar to those with HFrEF when guideline recommended therapies were used.11,13,25–27
In the CHART-2 study,13 prognostic impacts of ACEis, ARBs, MRAs, beta-blockers, statins, calcium channel blockers and diuretics in HFmrEF patients were different from those in HFpEF patients, but were almost comparable to those in HFrEF patients.13 The use of beta-blockers was positively associated with improved mortality in HFmrEF and HFrEF, but not in HFpEF patients.13 Diuretics had a negative prognostic impact in HFmrEF and HFrEF, but not in HFpEF patients, whereas statin use was associated with reduced mortality in HFpEF, but not in HFmrEF or HFrEF.13
Similar findings were obtained in the analysis of the Swedish Heart Failure registry,11 beta-blocker therapy was associated with reduced 1-year mortality in HFmrEF with CAD (HR=0.74, p=0.01) but not in HFmrEF without CAD (HR=0.99, p=0.94). ACEis and ARBs were effective in reducing the risk of death regardless of the presence or absence of CAD (HR=0.67 and HR=0.59, respectively). It should be noted that in this study diuretics demonstrated a negative impact on survival in HFmrEF patients.
The benefit of beta-blockers in HFmrEF patients was confirmed in an individual patient-level analysis of 11 major double-blind randomised trials.24 Beta-blockers improved mortality in sinus rhythm but not in patients with atrial fibrillation in all EF categories except LVEF ≥50 %.
The post hoc analysis of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial (TOPCAT)25 revealed a reduction in the primary endpoint (a composite of death from cardiovascular causes, aborted cardiac arrest, or HF hospitalisation) in HFpEF patients on the lower end of the EF spectrum – LVEF 45–49 %, but not when LVEF was above 60 % (LVEF <50 %: HR=0.72; LVEF ≥60 %: HR=0.97, p=0.046).
The recently-published post hoc analysis of the CHARM Programme (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity)26 determined a similar statistically significant improvement in the primary outcome (cardiovascular death or HF hospitalisation) for candesartan versus placebo both in patients with HFrEF and HFmrEF (HR=0.82, p<0.001 and HR=0.76, p<0.02, respectively), but not in HFpEF (HR=0.95, p=0.57).
Interestingly, a reduction of NT-proBNP levels in patients with HFmrEF during routine care was associated with a lower risk of all-cause death or HF hospitalisation.28 However, whether NT-proBNP changes will predict drug efficacy is yet to be clarified.
Currently, new prospective, adequately designed and powered studies that would confirm the benefits of modern HF therapies in patients with HFmrEF are needed. The most pragmatic approach appears to be an analysis of treatment effects across the entire EF spectrum, especially in patients with EF <50 % (HFrEF and HFmrEF). Furthermore, such an underlying cause of HFmrEF as CAD will have to be taken into account because the importance of pharmacological and invasive strategies for improving prognosis in this category of patients is well known.
Conclusion
It can be already argued that the introduction of HFmrEF as a separate phenotype of HF has achieved its aim of drawing attention to HFmrEF, given the increased number of published studies. The comparison of the clinical characteristics, comorbidities, outcomes and prognosis among patients with HFpEF, HFmrEF and HFrEF allowed consideration of HFmrEF as an intermediate phenotype, which often resembles HFrEF more than HFpEF. Moreover, HFmrEF can dynamically transit into HFpEF or HFrEF, suggesting that HFmrEF represents a transitional status between HFpEF and HFrEF rather than an independent entity of HF. Much less is known about the underlying pathophysiological mechanisms of HFmrEF. New studies are needed not only to improve our understanding of the pathophysiology of HFmrEF, but also to identify effective therapeutic strategies. The latest findings on the beneficial effects of therapies for HFrEF in HFmrEF patients are promising.







