Heart failure (HF) is a complex syndrome caused by functional and structural abnormalities of the left ventricle (LV) resulting in a combination of typical signs and symptoms. Historically, HF has been classified according to LV ejection fraction (EF) as either HF with reduced EF (HFrEF; LVEF <40%) or HF with preserved EF (HFpEF; LVEF >50%).1,2 The 2016 European Society of Cardiology (ESC) guidelines on acute and chronic HF established an HF category of ‘HF with mid-range ejection fraction’ (HFmrEF), defined as EF between 40% and 49% in patients with HF, to promote research into the main characteristics of this separate group of patients.3 In recent years, increasing evidence has emerged that HFmrEF may represent a subgroup of HF patients with a peculiar clinical, biomarker and diagnostic profile. However, a considerable number of HFmrEF patients experience improvement in LVEF, even to normal values. Therefore, whether this is a unique subtype of HF patients or whether it represents a ‘transition phase’ from HFrEF to HFpEF or vice versa is still a matter of debate.
This article focuses on the epidemiology, clinical characteristics and therapeutic approaches to HFmrEF with the aim of discussing the major determinants of transition to preserved or reduced EF.
Epidemiology
Extensive data are lacking about the prevalence of HFmrEF because there have been no population-based clinical studies and most epidemiological studies have divided HF patients into two groups using an EF cut-off value of 50%. Therefore, to obtain putative epidemiological information, we rely on subanalyses of clinical registries and on investigations that report the prevalence of different EF values within the populations under investigation.
A recent analysis of the Get With The Guidelines-HF (GWTG-HF) registry, which provides data on almost 100,000 patients hospitalised acute HF from 2005 to 2013, found that HFmrEF accounted for 13% of cases.4 In the Swedish HF registry, 21% of all hospitalised HF patients had HFmrEF.5 In the US, HFmrEF has been reported to account for 13–24% of patients with HF.6 Finally, the Chinese HF registry reported a prevalence of HFmrEF of 26.6% within the HF population, with no differences in trends between urban and rural areas.7
HF phenotypes do not unusually represent transitory stages due to fluctuations in LV volumes and systolic function. Research has increasingly confirmed that, among all HF phenotypes, EF variations are most common in HFmrEF. Transition from HFmrEF towards preserved LVEF has been reported in 25–44% of patients, and towards reduced LVEF in 16–33% of patients.5,8,9
Mesquita et al. subdivided the HFmrEF population into three different categories according to LVEF transition, namely recovered HF (73%; from HFrEF to HFmrEF), impaired HF (17%; from HFpEF to HFmrEF) and unchanged (10%; showing no changes in EF during follow-up).10
These data clearly point to a need for close clinical follow-up in order to determine whether the current HFmrEF phenotype in the patients we are examining represents a stable stage of mild disease or a transitional step towards different LVEF levels.
Diagnosis of HFmrEF: Role of Multimodality Imaging
It is imperative to keep in mind that, as an endocardial measurement, LVEF quantification is disproportionately influenced by loading conditions and by chamber geometry, and has important limitations in identifying subclinical LV dysfunction.
Echocardiography remains the most common modality for both LVEF measurements and analysis of sequential variability during time. However, due to the possibility of suboptimal views and the presence of a myocardial ischaemic area or other factors that make it difficult to correctly identify the endocardium, 2D biplane echocardiography is believed to be less accurate than other techniques, including 3D echocardiography and global longitudinal strain (GLS) for measurements of both ventricular volume and EF.11–13
GLS, a direct measurement of myocardial fibre deformation, can contribute to the identification of residual LV impairment despite normal or near-normal LVEF. In HF patients with recovered LVEF, an abnormal GLS predicts the likelihood of decreasing LVEF at follow-up and is associated with a significantly worse outcome than that seen in patients with normal GLS values.14,15 Moreover, HFpEF patients with reduced GLS (less than –14%) represent a high-risk group that could slide towards clinical instability and reductions in LVEF.16
Cardiac magnetic resonance imaging (CMR) is another tool that, through its high spatial resolution and multiple imaging modalities, can guide physicians in evaluating LV status. Recent studies have confirmed the roles of CMR in predicting LVEF deterioration through analysis of late gadolinium enhancement and in uncovering undetected cardiac pathology in HFpEF.17,18 Finally, elevated T1 and T2 relaxation times are typical of HFmrEF patients relative to healthy controls, and could be instrumental in predicting increasing fibrosis and inflammation despite normal LVEF.19
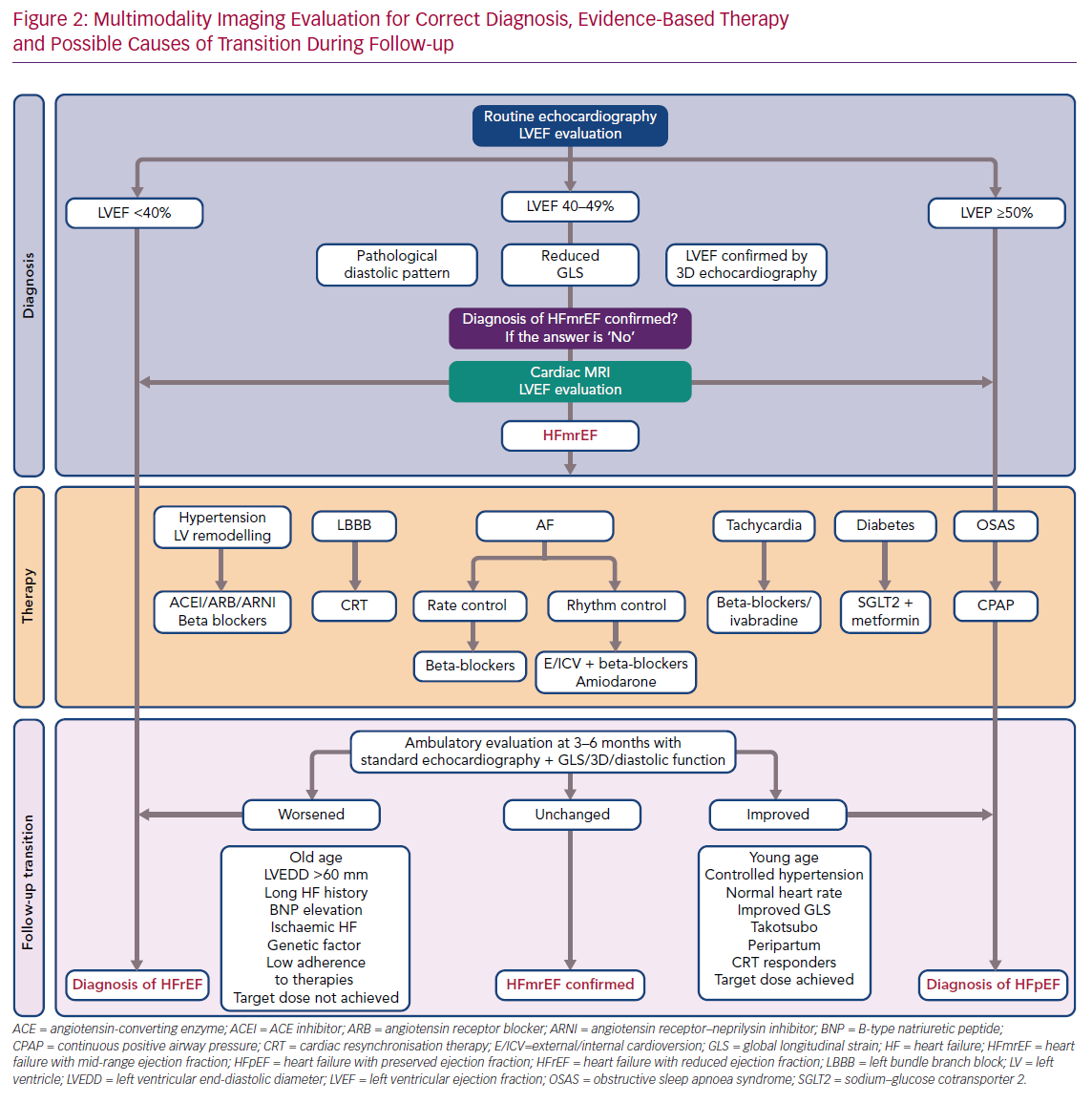
It has been suggested that a complete echocardiographic examination, including 2D and 3D echocardiography, GLS and analysis of diastolic dysfunction, should be performed to characterise HFmrEF in HF patients. In cases in which borderline data have been obtained, or results from different examinations are contradictory, ventricular function should be analysed using CMR.
Clinical Features of HFmrEF
Most clinical information on HFmrEF has been derived from comparative investigations with HFrEF and HFpEF. In fact, given the low prevalence of HFmrEF compared with that of HFrEF or HFpEF, it may not be feasible, despite being scientifically desirable, to obtain a considerable number of trials focusing on HFmrEF as a separate population.
With regard to cardiovascular risk factors, the prevalence of hypertension in HFmrEF ranges from 60% to 82%, which is higher than in HFrEF, but lower than in HFpEF.4,5,20,21 Diabetes is also common in HFmrEF, with a prevalence ranging from 28% to 48%, which is comparable to the prevalence of diabetes in HFpEF but higher than the prevalence in HFrEF.4,5,20,22–24
The Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program found that HFmrEF patients more closely resembled HFrEF patients in terms of age and sex.2 Conversely, Kapooer et al., in a study based on the GWTG-HF Registry, reported that HFmrEF patients were older (mean age 77 years) and a higher proportion were female (48%) compared with HFrEF patients.4 Of note, patients with HFmrEF seem to be mostly male and younger than those with HFpEF.23,24
The prevalence of non-cardiac comorbidities, in particular chronic obstructive lung disease, anaemia and renal insufficiency, in HFmrEF is intermediate compared with HFrEF and HFpEF.4,5,25,26 Data from the Swedish HF registry analysed the interaction between comorbidities and prognosis, finding that coexisting chronic kidney dysfunction and AF increased the risk of cardiovascular events to a significantly greater extent in HFmrEF than in HFrEF or HFpEF.27
Cardiac ischaemic disease appears to be common in patients with HFmrEF: in fact, an ischaemic comorbidity burden higher than in HFpEF but similar to that in HFrEF has been reported for HFmrEF almost worldwide.4,5,20,24,28,29 This relatively high amount of concordant data on the higher prevalence of ischaemic heart disease in HFmrEF than in HFpEF suggests that a significant percentage of HFmrEF patients may represent a group in the early phase after an ischaemic event, a clinical picture that is similar to that of HFpEF but enhanced by a significant ischaemic burden.
Medical Therapy
The main controversies regarding therapeutic options in HFmrEF include which therapy is best and how to decide whether to maintain, modulate or interrupt medical treatment in HFmrEF patients.
The CHARM study showed that candesartan may reduce cardiovascular (CV) and HF events in HFmrEF (7.4 versus 9.7 per 100 patient years; HR 0.76; 95% CI [0.61–0.96]; p=0.02).22 Similar findings have been reported in studies of beta-blockers. For example, Cleland et al. reported a reduction in CV death in HF patients in sinus rhythm and with LVEF between 40% and 49% treated with beta-blockers compared with placebo (HR 0.48; 95% CI [0.24–0.97]; p=0.04) and improved LV systolic function.30 The Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) study demonstrated a decrease in HF hospitalisations in patients treated with spironolactone, with the greatest benefit observed in those with LVEF ranging from 45% to 55%.31 In another recent study, spironolactone use at discharge after an acute HF episode was associated with better long-term outcomes.32 A retrospective analysis of the Digitalis Investigation Group (DIG) suggested a slightly higher decrease in CV death and HF hospitalisation in HFmrEF than HFpEF patients.33
However, the most fascinating question that remains unanswered is whether specific treatments in HFmrEF patients could be associated with the transition to preserved or reduced LVEF.
Predictors of LVEF Transition
LVEF transition in HF patients is a critical turning point because it may represent a spontaneous or therapy-induced improvement in the natural history of the disease, and therefore in the prognosis, or a possible point of no return towards a decline in the condition.34 For this reason, it is mandatory that the clinical picture of every HFmrEF patient seen is carefully examined so that the evolution of the condition can be predicted as much as possible. Indeed, because HF classification is derived from an artificial arbitrary construct based on EF thresholds, patients in the HFmrEF cohort are part of a continuum of known heterogeneous pathophysiologies.
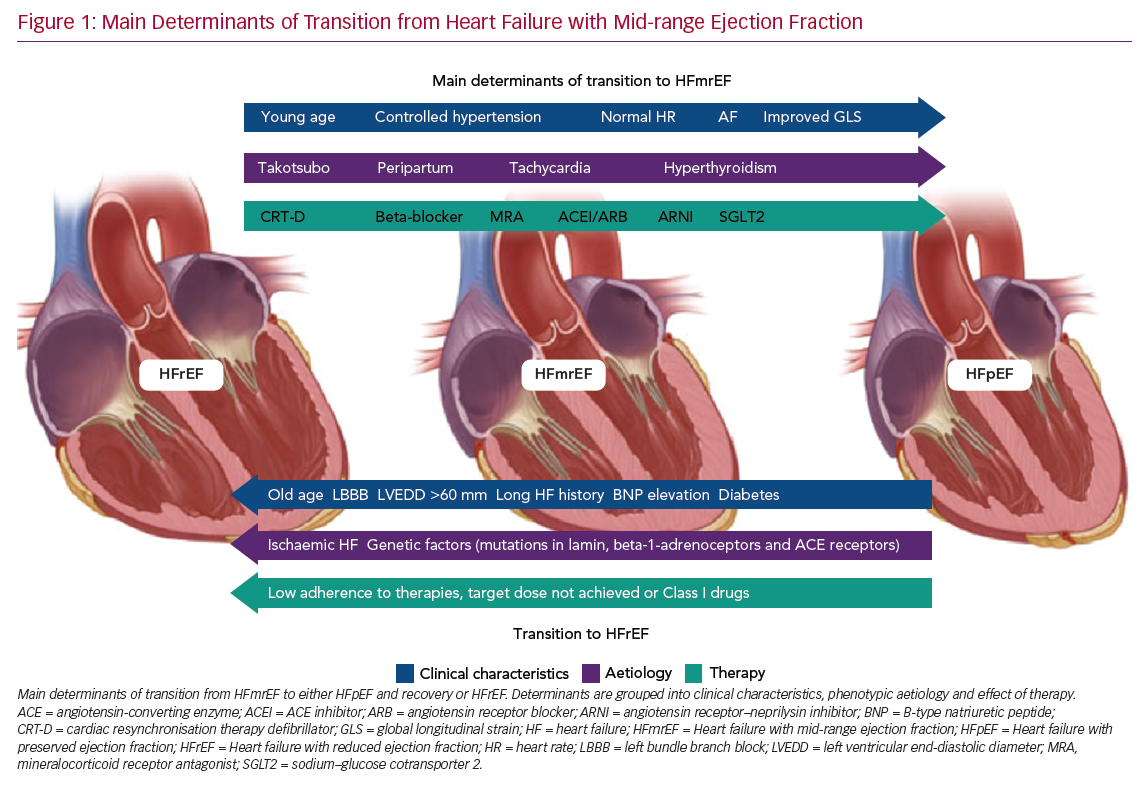
Predictors of transition from HFmrEF towards either HFpEF (and recovery) or HFrEF are shown in Figure 1.
Predictors of LVEF Improvement
It is clear that the frequency and degree of LVEF improvement depend primarily on the cause of HF. For example, patients with Takotsubo cardiomyopathy often present a rapid improvement towards normal LVEF values, even in the absence of medical therapy, and have a good long-term prognosis.35,36 Patients with acute myocarditis, who survive the critical phase, frequently exhibit LVEF recovery and excellent outcomes.37,38 Peripartum cardiomyopathy, once excluded from the group of genetically determined dilated cardiomyopathies, is another type of HF that has a satisfactory rate of recovery towards normal LVEF values.39
Significant rates of LVEF improvement to values in the preserved range have been reported when considering causes of recent onset (<6 months) HF, such as tachycardia- and hyperthyroidism-induced cardiomyopathy.40
Similarly, the duration of HF is a primary factor predicting HF transition: in both HFmrEF and HFrEF, patients with long-standing HF show low rates of LVEF improvement.41
Systemic hypertension, AF, lower NYHA functional class (I–II) and younger age (<65 years) have been reported as clinical features associated with LVEF recovery to preserved values.42–44 It seems reasonable to suggest that specific treatment of comorbidities should be addressed first, whenever possible. It is also clear that in patients with HFmrEF, uncontrolled hypertension is one of the main determinants of hospitalisation for HF: therefore, extended use of angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors from the early phase of the disease may reduce the risk of LVEF decline.
Cardiac resynchronisation therapy (CRT) is a milestone treatment in HFrEF, with a significant proportion of patients showing improvements in LVEF to values comparable with the phenotype of HFmrEF following implantation of a CRT device, with only a minority, the so called ‘super-responders’, showing improvements in LVEF to values >50%.45,46
This highlights two important factors, namely the main role of LV dyssynchrony in fuelling LV deterioration and the importance of HF therapies in reversing different HF phenotypes according to individual patient’s responses and their adherence to available therapies. Some patients who exhibit left bundle branch block (LBBB) improve less on optimal medical therapy alone and often present mid-range EF.47 This association of LBBB and HFmrEF raises the question as to whether this cohort of patients may benefit significantly from expanding the indications for implantation of a CRT device to LVEF >35%, particularly in the absence of reasons other than LBBB itself as the cause of LV dysfunction.48
It has been suggested that patients with a history of HFrEF and a subsequent improvement in LVEF, which reclassifies them as HFmrEF, should be treated by maintaining all HFrEF therapies at the maximum tolerated dose.49 In particular, an important step towards clinical and prognostic improvement has come from the introduction of new therapeutic options, namely an angiotensin receptor–neprilysin inhibitor (ARNI) and sodium–glucose cotransporter 2 (SGLT2) inhibitors.
Many studies have shown improvement in the main echocardiographic parameters in patients with HFrEF after the introduction of ARNI.50,51 The benefits in terms of prognosis, rehospitalisation and quality of life offered by ARNI suggest that therapy initiated at the maximum tolerated dose, during the HFrEF stage, has to be continued at the same dosage, and should be down-titrated only if acute kidney failure or severe hypotension occur.52,53 In the Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF) trial, ARNI failed to reduce the primary composite endpoint of total hospitalisations for HF and CV death in patients with HF and LVEF ≥45%.54 However, when data from Efficacy and Safety of LCZ696 Compared to Enalapril on Morbidity and Mortality of Patients With Chronic Heart Failure (PARADIGM-HF; eligibility criterion LVEF ≤40%; n=8,399) and PARAGON-HF (eligibility criterion LVEF ≥45%; n=4,796) were combined in a prespecified pooled analysis, the therapeutic effects of sacubitril/valsartan (ARNI) compared with a renin–angiotensin system inhibitor alone varied according to LVEF, but treatment benefits, particularly for heart failure hospitalisations, appeared to extend to patients with HFmrEF.55
The attention to HF care in patients with type 2 diabetes (T2D) has increased markedly after results were published from three randomised clinical trials evaluating the effects of SGLT2 inhibitors.56–58 Three different SGLT2 inhibitors have been demonstrated to prevent the development of HF and prolong life in patients with T2D. More recently, data have been presented for patients with HFrEF and with and without T2D. In that study, patients who received the SGLT2 inhibitor dapagliflozin had a lower risk of worsening HF or death from CV causes and better symptom scores than those who received placebo, regardless of the presence or absence of diabetes.59 Although at the present time we lack specific studies in the HFmrEF population, SGLT2 inhibitors represent a promising class of drugs for the treatment of HF in the future.60
Predictors of LVEF Deterioration
Among 174 HF patients with EF ≥45% who were on beta-blockers and were followed for 4–10.8 years, older age (mean [±SD] 56 ± 12 years), lower heart rate (59 ± 9 BPM), the presence of complete LBBB and a larger LV end-diastolic diameter (60 ± 7 mm) were reported as independent predictors of LVEF deterioration.61
LVEF deterioration and the absence of a transition towards recovered HF have been associated with an ischaemic aetiology of HF and with comorbid diabetes.43,62 These results, together with the reported connection between both a non-ischaemic origin of HF and the absence of diabetes and LVEF improvement, strongly suggest the importance of early, aggressive treatment of diabetes and ischaemic heart disease in order to defuse their detrimental effects on the benefit of optimised HF therapy.63
Recent studies have proved that genetic factors can help physicians predict the evolution of LVEF and HF. In fact, dilated cardiomyopathies with lamin mutations, certain polymorphisms in beta-1-adrenoceptors and angiotensin-converting enzyme receptors are all associated with progressive LV dysfunction in chronic HF patients.64 Moreover, physicians need to be aware that HFmrEF resulting from deterioration of LVEF previously >50% is associated with a higher risk of all-cause mortality and hospitalisation, as reported recently.65
In summary, certain clinical characteristics may help predict LVEF transition. However, none of these factors has a predictive value high enough to suffice as a prognostic tool on its own. Furthermore, classification efforts are hampered by a lack of homogeneity in study populations for the characteristics mentioned above. Therefore, as research continues into the mechanisms underlying LVEF variation, we believe that a tailored approach to individual patients that encompasses HF aetiology, duration, comorbidities, response and adherence to therapies is the optimal way to manage these patients (Figure 2).
Data From the Italian Network on Congestive Heart Failure Registry
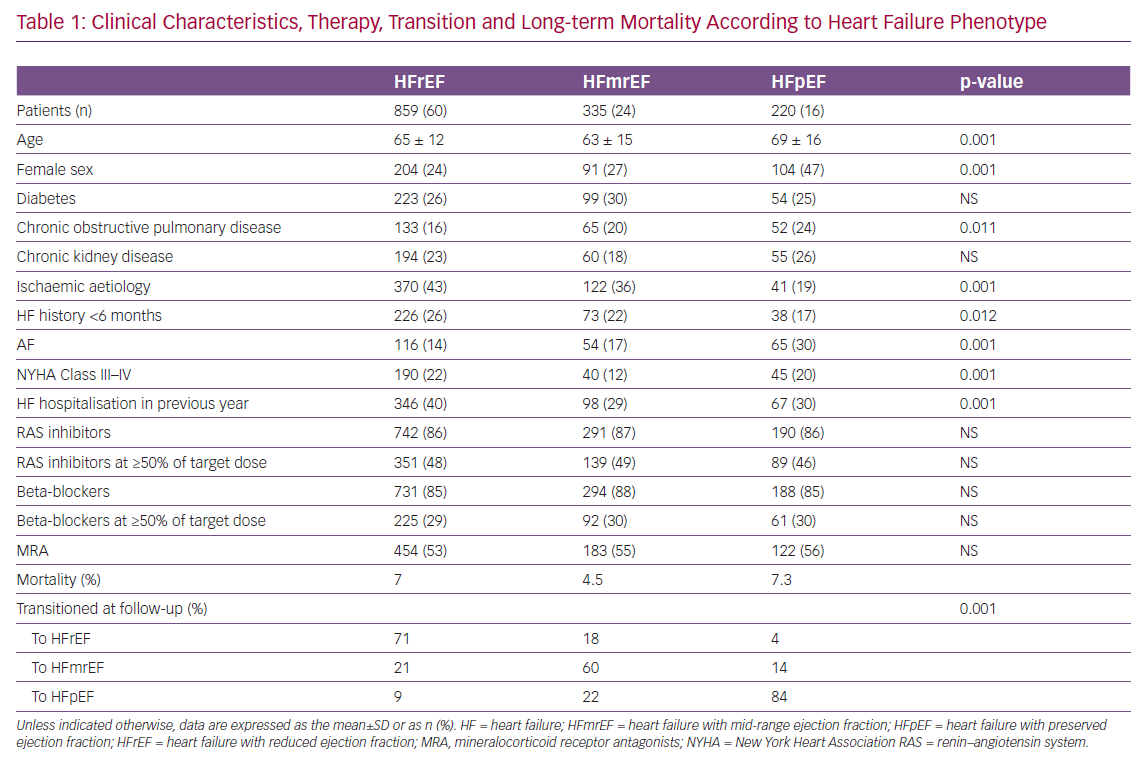
Of the 7,559 congestive heart failure (CHF) patients enrolled between 2009 and 2016 in the Italian Network on Congestive Heart Failure (IN-CHF) registry,66 data were analysed for 1,414 who had a second echocardiographic examination during a phase of clinical stability at a median time of 6 months since recruitment. Patients were classified according to baseline EF as either HFpEF (n=220), HFmrEF (n=335) or HFrEF (n=859). Clinical characteristics, therapy, transition to a different HF phenotype and long-term mortality are reported in Table 1. HFmrEF patients were more similar to HFrEF than HFpEF patients and, during follow-up, showed greater variability than patients in the other two groups. At the second echocardiographic examination, only 60% of patients with HFmrEF at the time of enrolment stayed in the same group (compared with 71% of HFrEF and 82% of HFpEF patients). When patients were reclassified according to EF at the follow-up echocardiogram, mortality after a mean (±SD) follow-up of 36 ± 28 months was 3.7%, 8.1% and 6.4% for patients in the HFmrEF, HFrEF and HFpEF groups, respectively (p=0.01).67
When considering EF changes over time in patients with HFrEF and HFmrEF, variables associated with reclassification to HFmrEF or full EF recovery at follow-up differed between baseline phenotypes.67 Multivariable logistic regression revealed that a lower likelihood of recovery was associated with ischaemic aetiology in both the HFmrEF (OR 0.66; 95% CI [0.19–0.68]) and HFrEF (OR 0.46; 95% CI [0.33–0.64]) groups, as well as with NYHA Class III–IV in the HFrEF group (OR 0.57; 95% CI [0.38–0.68]). Conversely, in the HFmrEF group, a history of HF <6 months (OR 2.44; 95% CI [1.76–3.39]) and AF (OR 2.66; 95% CI [1.37–5.17]) independently predicted phenotype transition or full recovery.67
These data suggest that clinical studies on HFmrEF should consider its peculiar temporal trend to possible transition into either HFrEF or HFpEF, with associated changes in mortality.
Prognosis
There is considerable published data concerning prognosis in HFmrEF. However, the information available regarding outcomes and prognosis does not apply to acute HF episodes. Moreover, whether HFmrEF patients obtain the same benefit from the disease-modifying drugs in the HFrEF armamentarium remains unclear.
Large registries report 1-year mortality of 7.6% for HFmrEF, which is intermediate between the 1-year mortalities reported for HFpEF (6.3%) and HFrEF (8.8%), and a 5-year mortality of 75% among HFmrEF patients aged ≥65 years.4,68 No difference in mortality between the three HF phenotypes was demonstrated in the Trial of Intensified (BNP-guided) versus standard (symptom-guided) Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) cohort after a median follow-up of 794 days.28 In the meta-analysis of Altaie et al., only a few outcomes differed between HF phenotypes: patients with HFmrEF had a lower rate of all-cause death than those with HFrEF, whereas patients in the HFpEF group had a higher rate of cardiac mortality than those in the HFmrEF group.69 With regard to sudden cardiac death, Pascual-Figaz et al. revealed a higher risk for HFmrEF than HFpEF patients (HR 2.73; 95% CI [1.07–6.98]; p=0.036).70 Conversely, non-cardiac mortality was higher among patients with HFrEF than HFmrEF.70 In the CHARM program, HFmrEF patients had a lower HRs for cardiac and all-cause mortality than HFrEF patients.22
Clearly, LVEF transitions have a crucial role in terms of prognosis: lower mortality, hospitalisation for HF and better functional capacity characterise HFmrEF patients moving towards HFpEF compared with those with HFrEF and HFmrEF who did not transition.71–73 A sharp decline in the composite outcome of death, LV assist device implantation or heart transplantation has been reported even in patients transitioning from HFrEF to HFmrEF compared with HFrEF or HFmrEF without LVEF recovery during follow-up.71
Conversely, decreases in LVEF are correlated with a worse outcome: HFmrEF transitioning to HFrEF is associated with higher mortality, rates of heart transplantation and hospitalisation for acute HF than HFmrEF with no decrease in LVEF.4,74
Nonetheless, HF patients with recovered LVEF should not be considered as ‘healed’, and a complete clinical examination is mandatory. In particular, abnormal concentrations of B-type natriuretic peptide (BNP), uric acid and troponin I, which denote persistent neurohormonal activation, increased oxidative stress and cardiomyocyte injury, have been associated with a persistent risk of hospitalisation for HF and clinical instability, despite LVEF recovery.41,75
These findings suggest that, despite improvements or recovery in LVEF, a continuous comprehensive evaluation through biomarkers and using modern imaging tools is needed in order to carefully plan the timing of clinical follow-up and the eventual long-term continuation of medical therapies.
Conclusion
Since its classification as a separate entity, it has become increasingly clear that HFmrEF mostly represents a transition phenotype, either to full recovery or to a downhill course of worsening systolic function. Differences in the prevalence of risk factors and underlying aetiology may generate different triggers of transition to either preserved or reduced EF, and thus different outcomes.
A multiparametric approach to accurately profile HFmrEF is needed to predict disease evolution; moreover, we stress the importance of considering complete multimodal imaging evaluation (i.e., 3D, GLS and eventually CMR) to carefully evaluate LVEF beyond the physiological and inherent technical limitations of 2D echocardiography. Evidence-based therapies, particularly for patients with EF <45%, and aggressive treatment of comorbidities are crucial for favourable transitions. Trials of future treatments need to take into account the highly dynamic nature of this peculiar phenotype.