Heart failure (HF) is a major healthcare problem associated with high rates of morbidity and mortality.1 Approximately 6 million Americans aged ≥20 years have HF, and it is the leading cause of hospitalisation among older adults with estimated healthcare costs of US$31 billion annually.1,2 HF with preserved ejection fraction (HFpEF) accounts for over 50 % of all HF cases, and unlike HF with reduced ejection fraction (HFrEF), pharmacological, or device-based therapy do not improve survival or quality of life in patients with HFpEF.3,4
The hallmark symptom of HFpEF, even when well compensated and non-oedematous, is impaired exercise tolerance, measured objectively as decreased peak aerobic power (VO2peak).5–8 Specifically,VO2peak is 35 % lower in patients with HFpEF versus age-matched healthy controls secondary to central and peripheral abnormalities that result in reduced oxygen delivery to and/or utilisation by the exercising muscles.7,9-12 A consequence of the reduced VO2peak is that basic (getting dressed) and instrumental (housework, walking to get mail) activities of daily living require near-maximal effort. Given that exercise intolerance is a major independent predictor of hospital readmissions and mortality in HFpEF, a major goal of therapy is to improve VO2peak in patients with HFpEF.13
Prior studies have demonstrated that exercise training is a safe and effective therapy to improve VO2peak, cardiovascular and skeletal muscle function, quality of life and hospital readmission rates in patients with HFrEF.14–24 Furthermore, exercise-based cardiac rehabilitation has been shown to modestly reduce both all-cause and cardiovascular mortality rates in HFrEF.19 As a result, in the US example, exercisebased cardiac rehabilitation is covered by Medicare in patients with HFrEF. In contrast, despite numerous studies showing that exercise training is a safe and effective therapy for improving exercise tolerance and quality of life in HFpEF,25–28 exercise-based cardiac rehabilitation is currently not covered by Medicare in patients with HFpEF due to limited data on its effect on survival in this population.
In this brief review, we discuss the role of exercise training to improve VO2peak and the central and peripheral adaptations that occur with physical conditioning in patients with HFpEF.
Effects of Exercise Training on Peak Aerobic Power in Heart Failure With Preserved Ejection Fraction
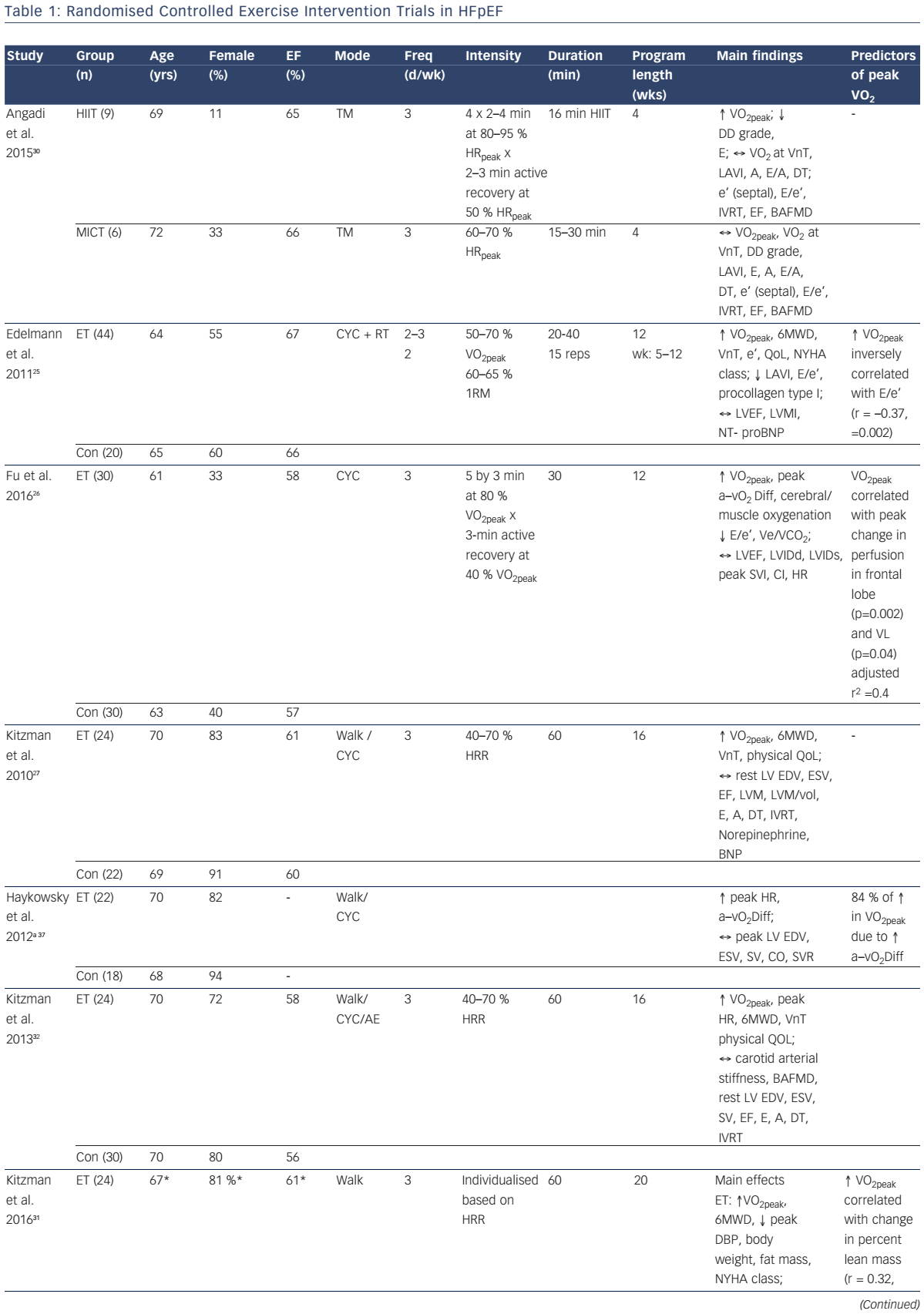
To date, eight randomised controlled trials have examined the efficacy of exercise training to improve VO2peak in patients with HFpEF (see Table 1).25–32 Specifically, in five of these studies, moderate-intensity continuous endurance training (MICT) was performed 3–5 days per week for 1–5 months. This exercise prescription is in line with the American Heart Association exercise guidelines for overall cardiovascular health, which recommends at least 30 minutes of moderate-intensity aerobic physical activity (such as walking) at least 5 days per week.33 Two studies incorporated high-intensity interval training (HIIT), consisting of 4–5 intervals performed at 80–95 % peak heart rate for 2–4 minutes interspersed with 2–3 minutes of active recovery, for 1–3 months. Finally, Alves et al.29 estimated VO2peak after 6 months of moderate intensity interval training (five 3-min sets of moderate-intensity [70–75 % maximal heart rate] treadmill walking or cycling interspersed with 1-min low-intensity active recovery) in patients with HFpEF.
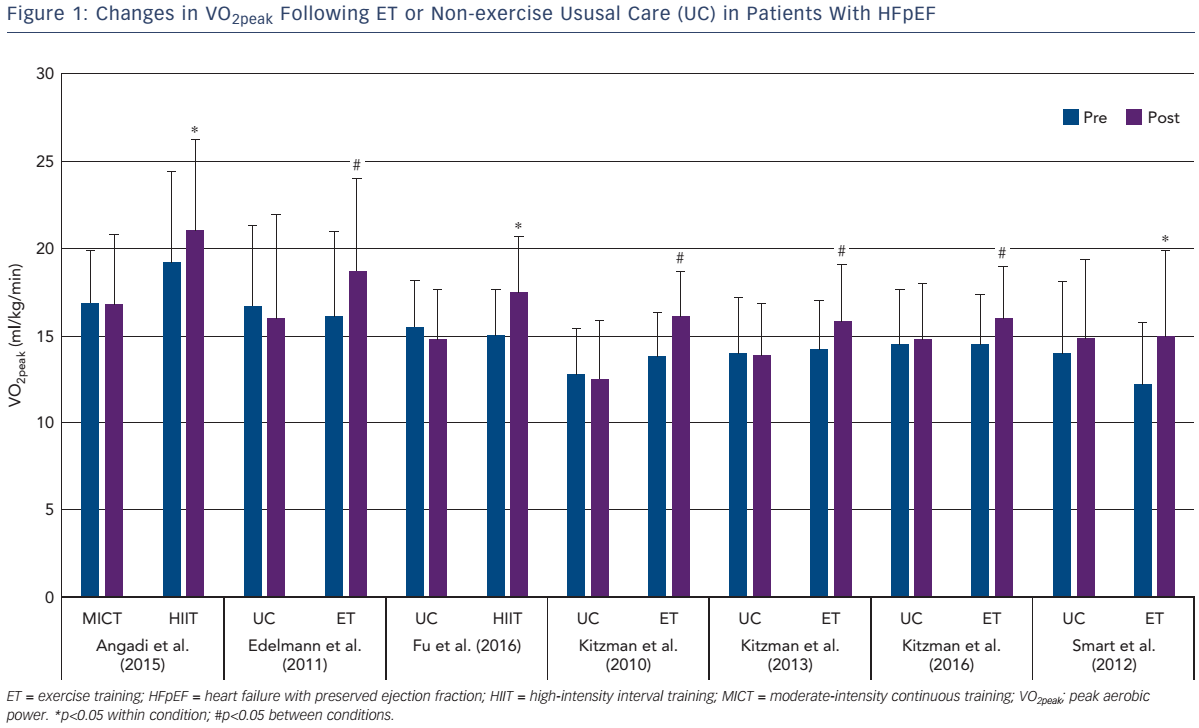
As shown in Figure 1, the increase in VO2peak after training is 2.2 ml/kg/min (~15 %), which is above the clinically meaningful change (1 ml/kg/min or 10 %) in VO2peak for patients with HF.34 The mechanisms responsible for the increased VO2peak may be due to cardiovascular or skeletal muscle adaptations that result in increased oxygen delivery and/or utilisation by the active muscles.
Exercise Training and Cardiac Function
The decreased VO2peak in HFpEF is due, in part, to impaired left ventricular (LV) relaxation, increased LV diastolic stiffness3 and decreased cardiac output secondary to blunted chronotropic, ionotropic and vasodilator reserve.5, 6,35 Several studies have assessed changes in resting and exercise cardiac function following exercise training in HFpEF. Kitzman et al.27,31,32 and others28,30 reported that MICT does not change resting LV volumes, morphology or systolic or diastolic function in patients with HFpEF (see Table 1). In contrast, Edelmann et al. reported that MICT and supplemental resistance training resulted in a significant decrease in resting left atrial volume index and the ratio of early mitral inflow velocity and mitral annular early diastolic velocity (E/e’).25 Moreover, the increase in VO2peak was inversely related to the improvement in E/e’. Fu et al. extended these findings by showing that HIIT significantly reduced the E/e’ ratio in patients with HFpEF.26 Although these non-invasive measures suggested that exercise training may improve LV filling pressure, Fujimoto et al. found that 1 year of progressive and vigorous endurance exercise training did not change LV compliance in older patients with HFpEF including during cardiac (un)loading manoeuvres.36
Currently, only two studies have compared exercise cardiac function before and after exercise training in patients with HFpEF.26,37 Using 2D echocardiography, Haykowsky et al. measured rest, peak and reserve (peak minus rest) LV volumes and cardiac output and estimated arterial– venous oxygen difference (a–vO2Diff) before and after 16 weeks of MICT in older patients with HFpEF.37 The training-mediated increase in VO2peak was secondary to a significant increase in peak and reserve heart rate and a–vO2Diff with no change in peak or reserve end-diastolic volume, end-systolic volume, stroke volume or cardiac output.
Using impedance cardiography, Fu et al. measured exercise cardiac output and estimated a–vO2Diff prior to and after 12 weeks of HIIT.26 Similar to the findings by Haykowsky et al.,37 the HIIT-mediated increase in VO2peak was secondary to the increased a–vO2Diff as peak heart rate, stroke volume index and cardiac index were unchanged after training.26 Accordingly, the increased VO2peak after MICT or HIIT appears to be due to non-cardiac peripheral adaptations that result in increased oxygen extraction by the active muscles.9
Exercise Training and Vascular Function
Recent evidence suggests that HFpEF is associated with both macrovascular38,39 and microvascular dysfunction,40,41 and that these vascular changes directly relate to exercise intolerance.9,38,39,42 First, patients with HFpEF demonstrate increased arterial stiffness compared with healthy controls as measured by carotid arterial and proximal thoracic aortic distensibility using high-resolution ultrasound and MRI.9,38,39 Second, patients with HFpEF have also been shown to have impaired microvascular function, as measured by laser Doppler imaging coupled with iontophoresis of endothelium-dependent (acetylcholine) and -independent (sodium nitroprusside) vasodilators.40 Together, these impairments are thought to contribute to reduced leg blood flow and leg vascular conductance during exercise.41,43
Interestingly, peripheral artery endothelial function is not significantly different in patients with HFpEF rigorously screened to exclude confounding effects of atherosclerosis compared with healthy agematched controls.42,44
Several investigators have examined the vascular effects of endurance exercise training in older patients with HFpEF. 30–32 Kitzman et al. first reported that carotid arterial stiffness and brachial artery flow-mediated dilation (FMD) were not significantly different after 8 or 16 weeks of MICT.32 More recently, Kitzman et al. found that 20 weeks of MICT did not change carotid–femoral pulse wave velocity (i.e. vascular stiffness).31 Angadi et al. confirmed and extended these findings by showing that brachial artery FMD was unchanged after 4 weeks of MICT or HIIT in older patients with HFpEF (see Table 1).30 Taken together, the few studies performed to date suggest that the benefits of MICT or HIIT likely derive from mechanisms other than alterations in central or peripheral vascular function in clinically stable patients with HFpEF (see Table 1). Future studies are required to examine the change in microvascular function following exercise training in HFpEF.
Changes in Skeletal Muscle Function With Exercise Training in Heart Failure With Preserved Ejection Fraction
Patients with HFpEF exhibit multiple skeletal muscle abnormalities, including increased skeletal muscle adiposity, reduced percent of type I (oxidative) fibres and enzymes, decreased capillary:fibre ratio compared with healthy, age-matched controls; these alterations are associated with their severely reduced exercise tolerance.44,45
There is a paucity of studies that have examined the effect of exercise training on skeletal muscle function. Haykowsky et al. demonstrated that 84 % of the increase in VO2peak after MICT was attributed to improved a–vO2Diff.37 Although the mechanisms of improved oxygen extraction were not studied, they may be due to favourable changes in skeletal muscle quality, as MICT does not change skeletal muscle mass.31 Indeed, Fu et al. recently reported that 12 weeks of HIIT significantly increased estimated peak exercise a–vO2Diff and vastus lateralis muscle oxygenation.26
No study to date has examined changes in capillary density, fibre type or oxidative metabolism within skeletal muscle following exercise training. However, pilot work by Bhella et al. using 31phosphate magnetic resonance spectroscopy demonstrated that leg muscle oxidative metabolism was reduced during exercise in a small number (n=2) of patients with HFpEF relative to healthy controls.46
Given that in patients with HFpEF skeletal muscle abnormalities are related to impaired exercise intolerance, future studies are required to examine the role of exercise (endurance and resistance) training to improve skeletal muscle morphology and oxidative capacity.47
Exercise Training-mediated Improvement in Peak Aerobic Power: Role of Training Intensity
HIIT is characterised by brief, intermittent bursts of vigorous aerobic exercise interspersed with periods of low-intensity active recovery. A recent meta-analysis comparing the effectiveness of HIIT versus MICT for improving VO2peak in HFrEF reported that HIIT is superior to MICT in improving VO2peak (weighted mean difference: 2.1 ml O2/kg/min).48 To date, only two randomised controlled trials have examined the effects of HIIT on VO2peak in HFpEF.26,30
Angadi and colleagues were the first to compare MICT versus HIIT on peak VO2 in older patients with HFpEF.30 The main finding was that HIIT resulted in a significant increase in peak VO2 (pre: 19.2 versus 21.0 ml/kg/min; p=0.04) with no change after MICT (pre: 16.9 versus 16.8 ml/kg/min; p=0.93).30 Fu et al. measured peak VO2 and its determinants after 12 weeks of HIIT in patients with HFpEF and HFrEF.26 Peak VO2 was significantly higher after HIIT; however, the mechanisms of improvement were dependent on underlying HF phenotype.26 Specifically, peak exercise heart rate, stroke volume index and cardiac index increased significantly with no change in peak a–vO2Diff in patients with HFrEF. Conversely, the increased VO2peak in patients with HFpEF was secondary to the increase in peak a–vO2Diff. Accordingly, the mechanisms responsible for the improvement in VO2peak may differ between patients with HFrEF and HFpEF.
In summary, short-term HIIT is an effective training stimulus to improve VO2peak; however, the magnitude of improvement (mean change: +2.2 ml/kg/min VO2peak) is similar to that found after MICT (mean change: +1.9 ml/kg/min VO2peak). Currently, it is unknown if HIIT is superior to MICT in increasing VO2peak in studies lasting >3 months; however, a large, multicentre randomised controlled exercise training intervention trial (OptimEx-CLIN study) is currently ongoing to examine the optimal dose and intensity of exercise training (12 months of HIIT versus MICT versus control) to improve VO2peak in patients with HFpEF.49
Novel Interventions in Heart Failure With Preserved Ejection Fraction
Fukuta et al. recently conducted a meta-analysis of randomised controlled trials on the effect of cardiovascular drugs (eight studies, 1080 patients) or exercise training (five studies, 245 patients) intervention on VO2peak, six-minute walk distance (6MWD) and quality of life (measured with the Minnesota Living with Heart Failure Questionnaire [MLHFQ] total score) in patients with HFpEF.50 The training mode included aerobic exercise (walking, cycling, 20–60 minutes per session, 2–3 days/week) for 12–24 weeks (a substantial portion of participants in the exercise intervention trials were receiving standard HF medications). Cardiovascular drug interventions included angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aldosterone antagonists or betablocker treatment (intervention duration ranged across trials between 14 and 52 weeks). The main finding was that exercise training improved VO2peak (weighted mean difference [WMD] of 2.3 ml/kg/min, 95 % confidence interval (CI) [1.3–3.2]), 6MWD (WMD of 30 min, 95 % CI [4.3–56.0]) and MLHFQ (8.9 points, 95 % CI [3.3–14.0]) compared with usual care. In contrast, cardiovascular drug intervention did not improve VO2peak, 6MWD or MLHFQ compared with placebo or no-treatment. More recent studies have examined the role of exercise training alone or combined with novel non-pharmacological therapy to improve exercise outcomes in patients with HFpEF.
In obese older adults without HF, weight loss via dietary caloric restriction (CR) improves LV mass and diastolic function, exercise capacity, glycaemic control, blood pressure regulation, body composition and skeletal muscle function.51–56 However, CR is controversial as a treatment for HFpEF, as observational studies suggest that overweight and moderately obese patients with HFpEF survive longer than those who are normal or underweight.57 Therefore, current HFpEF management guidelines do not include diet or CR.58
Kitzman et al. recently compared the effects of 20 weeks of CR or endurance training alone, or in combination, on VO2peak and quality of life in older obese patients with HFpEF (see Table 1).31 Endurance exercise training (+1.2 ml/kg/min VO2) and CR (+1.3 ml/kg/min VO2) significantly increased VO2peak and 6MWD, while the change in VO2peak with combined endurance training and CR were additive (+2.5 ml/kg/min). Both exercise training and CR also reduced body weight and fat mass, while CR improved leg muscle quality and decreased abdominal and thigh subcutaneous fat. Finally, the change in VO2peak was positively correlated with the change in percent lean mass and the change in thigh muscle to intermuscular fat ratio. Further studies are needed to determine whether these favourable changes in body composition are associated with reduced clinical events in patients with HFpEF.
Dietary inorganic nitrate supplementation, typically in the form of beetroot juice, has emerged as a novel strategy to lower blood pressure, and improve vascular health and exercise performance in patients with hypertension or HF.59–62 Inorganic nitrate, through its conversion to inorganic nitrite, targets nitric oxide delivery to areas of low oxygen content and may be a potential mediator of hypoxic vasodilation for exercising skeletal muscle.62 Furthermore, beetroot juice has been shown to improve exercise efficiency with multiple studies showing less oxygen consumed per unit of work performed.63,64
Zamani et al. performed a randomised, double-blind crossover study comparing a single dose of inorganic nitrate (12.9 mmol beetroot juice) with an identical nitrate-depleted placebo in 17 patients with HFpEF. 62 Beetroot juice increased VO2peak, total work performed and peak exercise cardiac output and reduced aortic augmentation index and systemic vascular resistance. Eggebeen et al. recently extended these findings by showing that 1 week of daily dosing with beetroot juice significantly improved submaximal aerobic endurance by 24 % and decreased resting blood pressure in older patients with HFpEF.59 These findings suggest that acute and short-term delivery of dietary inorganic nitrate can increase VO2peak and submaximal aerobic endurance in patients with HFpEF. Future, large-scale clinical trials are needed to determine whether dietary inorganic nitrate alone or combined with MICT or HIIT improve VO2peak and its determinants.
Training Effects in Related Populations
A special mention should be made of a recent observational study by Pathak et al. that demonstrated the efficacy of exercise training in obese patients with atrial fibrillation (AF; LV ejection fraction >40 %, mean average across groups E/E’ ratio = 12.1 and left atrial volume index = 39 ml/m2).65 Although patients with HFpEF were not independently identified in this study, the description of the cohort suggests that many patients likely met criteria for HFpEF. Among patients with obesity and AF, HFpEF is disproportionately represented, as are overlapping risk factors such as hypertension and diabetes (observed in ~75 % and ~25 % of the study cohort, respectively). It is also notable that approximately three-quarters of the cohort had an exercise capacity below expected norms and other measures of diastolic function were frequently abnormal. Thus, it may be reasonable to consider this study cohort under a broad umbrella of HFpEF.
Pathak et al. employed a novel intervention that included a tailored exercise programme that incorporated a combination of aerobic and strength exercises (increasing ≥200 min of exercise per week) as part of a dedicated physician-led risk factor management clinic that aggressively treated hypertension, hyperglycaemia, dyslipidaemia, obstructive sleep apnoea and obesity.65 A favourable response to exercise training was defined as an increase in maximal exercise capacity of more than two metabolic equivalent of tasks (approximating >20 % improvement) and this was achieved in 127 of the 308 subjects (41 %). This impressive intervention was associated with multiple health benefits including a marked reduction in AF, an improvement in diastolic measures and a reduction in left atrial size. In the absence of clear evidence as to which component of the lifestyle and risk factor intervention was most crucial, this key study reinforces the importance of taking a holistic view to cardiovascular health in addition to exercise training in patients with HFpEF or related risk factors.
Conclusion
Patients with HFpEF have severe exercise intolerance secondary to impaired cardiovascular and skeletal muscle function. The few randomised controlled exercise intervention trials performed to date demonstrate that endurance training alone or combined with resistance training is an effective therapy to increase VO2peak secondary to peripheral ‘non-cardiac’ factors that result in increased oxygen extraction by the active muscles. Unlike HFrEF, the mean change in VO2peak with HIIT (2.2 ml/kg/min) is similar to that found after MICT (1.9 ml/kg/min). Finally, exercise training combined with novel non-pharmacological interventions (CR or acute or 1 week of beetroot juice) has also been shown to improve VO2peak and aerobic endurance in patients with HFpEF, while broad-based risk factor interventions may enhance the benefits of exercise.