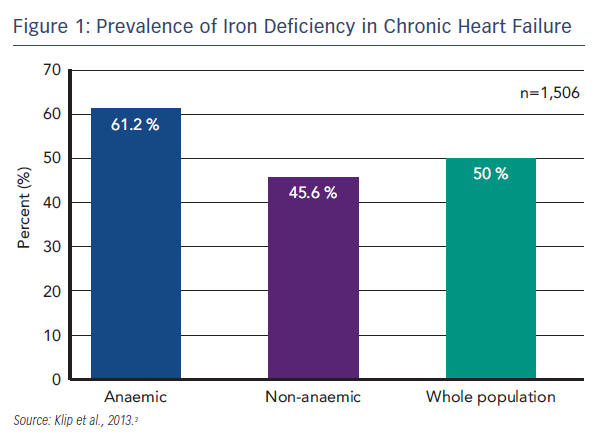
HF has a significant impact on QoL that is worse than the impact of other chronic diseases, particularly in terms of physical function.1 HF is characterised by exercise intolerance, fatigue and dyspnoea, and is classified according to severity in New York Heart Association (NYHA) classes I–IV, where Class I is no limitation of physical activity and Class IV is the inability to undertake any physical activity without discomfort.2 An emerging problem in HF is ID. ID is prevalent among patients with HF; in a recent international pooled cohort study (n=1,506), ID (defined as serum ferritin <100 µg/L or <299 µg/L if transferrin saturation [TSAT] <20 %) was found in 50 % of the total patient population. ID is the commonest cause of anaemia, but even in the absence of anaemia, ID was present in 45.6 % of patients (see Figure 1).3 Disease severity, assessed by NYHA class and N-terminal of pro-brain natriuretic peptide (NT-proBNP) levels, proved to be powerful and independent predictors of a disordered iron status. Furthermore, ID has been found to be an independent factor associated with reduced exercise capacity,4 reduced QoL5,6 and poor outcome.3
In 2012, the ESC Guidelines for the diagnosis and treatment of acute and chronic HF recognised ID as a co-morbidity in HF for the first time and recommended diagnosis of ID based on iron parameters in all patients suspected of having HF.2,7 Furthermore, the guidelines now detail the mechanism of action of iron in muscle function (and therefore the explanation for deficiency-related pathology and onset of symptoms in HF independent of the pro-erythropoietic function of iron); the need for routine monitoring for ID; and the beneficial effects on symptoms,
physical performance and QoL of treating ID with intravenous (i.v) ferric carboxymaltose (FCM). Based on the findings of the Ferric Carboxymaltose Assessment in Patients With IRon Deficiency and Chronic Heart Failure (FAIR-HF) study, which found that treatment with i.v. FCM in iron deficient patients with chronic HF improves symptoms, exercise capacity and QoL irrespective of whether anaemia was present or not. FCM is now considered as a possible treatment option in the current ESC Guidelines for HF.2,7,8 In conclusion, ID is a significant burden in HF and merits further investigation.