Heart failure (HF) is a leading cause of morbidity and mortality worldwide, affecting 1–2 % of the adult population in western countries with incidence of 5–10 per 1000 persons per year.1,2 It is estimated that the prevalence of HF will continue to increase as the population ages and, according to the American Heart Association (AHA), by the year 2030 the prevalence of HF in the US alone will rise to over 8 million patients, representing a 25 % increase compared to the
year 2010.3 HF is one of the common causes for hospitalisation, representing 1–2 % of all hospital admissions and the leading reason for admission in individuals above 65 years of age.4–6
HF is a progressive disease7 with approximately 5 % of HF patients suffer from end-stage disease refractory to medical therapy.8,9 The Heart Failure Association of the European Society of Cardiology (ESC) defined advanced HF as a state in which patients have significant cardiac dysfunction with severe HF symptoms, such as dyspnoea and/or fatigue, occurring at rest or with minimal exertion (NYHA functional class III or IV) despite maximal medical and device (cardiac resynchronisation therapy) therapy.10 In addition to the aforementioned symptoms, patients with advanced HF usually have objective measurements of peak VO2 (oxygen uptake) <14mL/kg/min, a 6-minute walk distance <300 meters, and poor cardiac function.10,11 The prognosis of patients with advanced HF is dismal, with life expectancy of less than two years.11,12 At this stage, advanced therapies are considered, including heart transplantation, continuous inotropic therapy, mechanical circulatory support or hospice.1,10,11 Heart transplantation remains the preferable therapy for advanced HF, but the number of transplants done worldwide is trivial compared to demand.13 Thus, durable mechanical circulatory support (MCS) devices have emerged as an important therapy for advanced HF.13,14
To date, over 18,000 continuous flow devices have been implanted worldwide.13,14 In the US alone, 131 hospital centres are approved to implant permanent MCS devices, demonstrating the staggering expansion of MCS as a therapeutic option for end-stage HF.15
The Nomenclature of MCS
A ventricular assist device (VAD) is a MCS device that is used to partially or completely support the function of a failing heart. Left ventricular assist devices (LVAD) pump blood from the left ventricle and transfer it to the ascending aorta. LVADs may be used as a bridge to transplant (BTT) for candidates awaiting heart transplantation; as destination therapy (DT) for patients who are not candidates for transplantation; as a bridge to decision for patients too sick to survive the transplant evaluation (so that their suitability for transplantation has not been determined at the time of VAD implantation) and as a bridge to recovery for selected patients who might recover their cardiac function. The latter patients are mostly those with acute cardiomyopathies (ie. fulminant lymphocytic myocarditis, peripartum cardiomyopathy, etc).11 Interestingly, according to the Sixth annual report of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) the proportion of patients treated with LVAD as DT in the United Sates has increased from 14.7 % in 2006–7 to 41.6 % in 2011–13.14
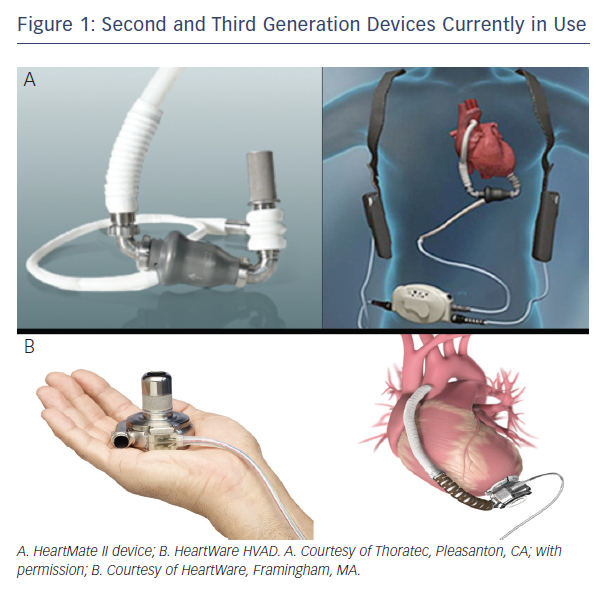
The first-generation VADs had pulsatile flow, designed to mimic the normal function of the heart. These devices were shown to increase survival and quality of life (QoL) of patients with end-stage HF compared to optimal medical therapy (OMT).16 The second and third generation devices currently in use (primarily HeartMate II, Thoratec Corp. and HVAD, HeartWare Ltd., see Figure 1), have continuous flow patterns, and can generate up to 10 litres/minute. Although these devices generate continuous flow, pulsatility may still be present in some patients since the flow delivered by the device is modified by native left ventricular (LV) contractility. Nevertheless, some studies suggest that pulsatile flow is not necessary for adequate perfusion of the end organs.17
In the ‘HeartMate II’ trial, treatment with continuous-flow HeartMate II devices as DT was associated with improved survival compared to
pulsatile-flow devices.18 In addition, patients treated with continuous flow-devices as DT had a significant reduction in the rate of adverse events and hospitalisations and had improved QoL and functional capacity compared to patients treated with pulsatile-flow devices.18
To evaluate whether ‘real life’ outcomes are similar to those observed in the clinical trials, Jorde et al.19 followed the first 247 patients treated with HeartMate II devices as DT who were not in a clinical trial and compared their outcome with those achieved
in the clinical trials. Survival in the later group trended to be better than in the initial clinical trial, with an absolute difference of 74 % versus 68 % at 1 year and 61 % versus 58 % at 2 years (p=0.2). Moreover, the rate of survival free of stroke (both haemorrhagic and ischaemic), device-related infection, or pump replacement was significantly higher in patients treated in the later group.19 These results are consistent with the outcomes summarised in the
Sixth INTERMCAS annual report of 80 % 1-year survival and 70 % 2-year survival.14
Treatment with the HeartWare HVAD device as a bridge to transplantation (BTT) was evaluated in the ADVANCE (HeartWare Ventricular Assist Device Bridge to Transplant) trial.20 In this study, the HeartWare HVAD device was compared to ‘commercially available devices’, mainly HeartMate II, in patients awaiting heart transplantation. The HVAD device was non-inferior to the HeartMate device with 1-year survival of 86 % and enhanced QoL and functional capacity similar to what was seen with the HeartMate II.20 The safety and effectiveness of HeartWare HVAD as DT is being evaluated in the ENDURANCE trial, yet to be published.
Patient Selection
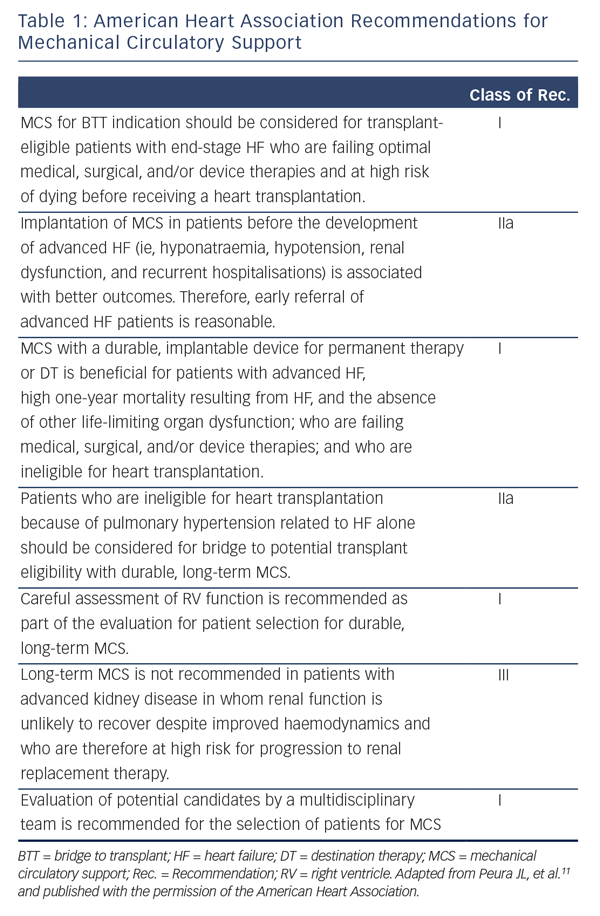
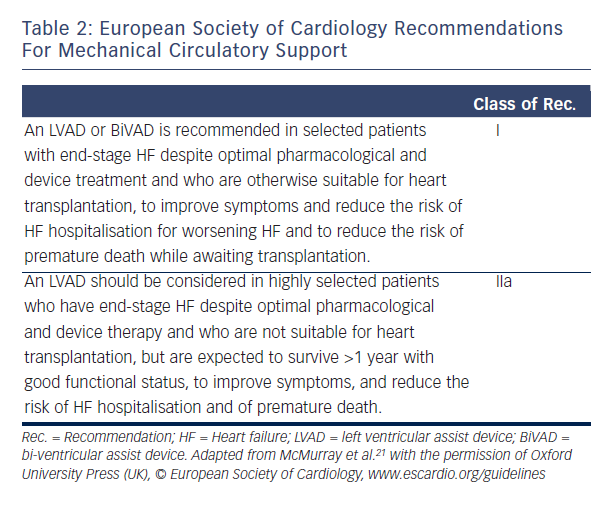
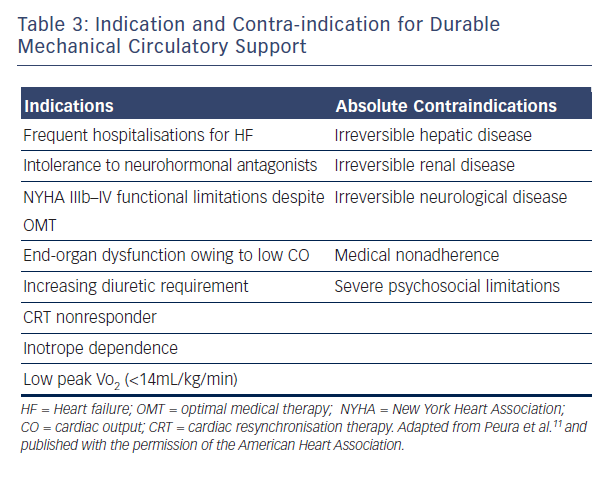
LVAD therapy should be considered in every patient with end-stage systolic (low LV ejection fraction) HF who has no other life-limiting diseases. Tables 1 and 2 summarise the current MCS recommendation from the AHA and the ESC. Table 3 details the indications and contraindication for MCS.
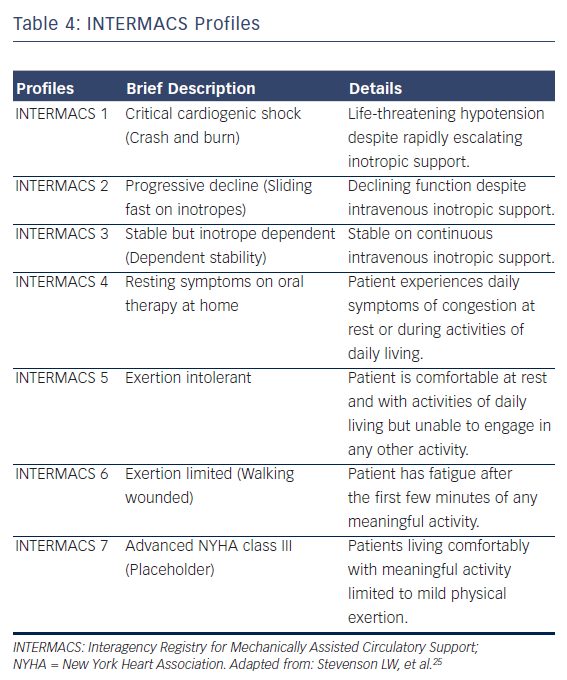
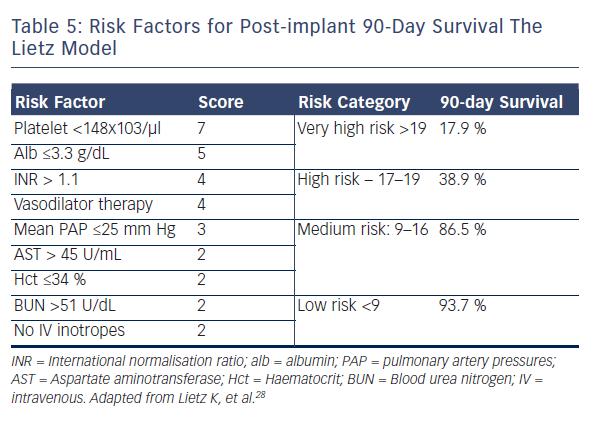
A MCS evaluation is essential to identify those patients who could benefit from device implantation, and to exclude those considered futile for device therapy. The first step in patient selection is to accurately estimate the clinical severity of the HF syndrome. Many US clinicians22 recommend the use of two prognostic scores, the Heart Failure Survival Score23 and the Seattle Heart Failure Model,24 to estimate the expected two-year survival on medical therapy in candidates who might benefit from LVAD support.22 The ESC recommend assessing the patient’s prognosis using variables that have been shown to predict outcome, such as findings in history and physical examination (NYHA class, blood pressure, signs of congestions, etc.), laboratory tests (serum sodium, liver enzymes, troponins, etc.), neuro-hormonal activity (Plasma renin activity, Angiotensin II, etc.), and functional (peak VO2) and haemodynamic variables.10,21 Likewise, it is now apparent that there are many phenotypes of advanced HF, which have been described with the INTERMACS profiles, a classification of 7 clinical profiles (see Table 4).25 Patients with INTERMACS profile 1 to 3 are being managed with temporary mechanical or inotropic support, whereas patients with profile 4 to 7 are not inotrope dependent.11,25 The INTERMACS profiles have been shown to provide prognostic information and guidance for the optimal timing and the associated risk of implantation.26,27 For example, INTERMACS profile 1 or 2 patients who are treated with LVAD have a 44 % higher post-implantation mortality than that of patients at INTERMACS profile 3 or 4.27 In addition, several risk scores have been developed for the estimation of short-term mortality after LVAD implantation.11,22 The ‘Lietz-Miller score’ was the most frequently used risk score for DT patients (see Table 5)28, but is now limited in use as it was developed on the first generation HeartMate XVE device.28
The second step in the evaluation is to search for significant co-morbidities and other factors that might limit the patient’s suitability.11 This search should include the possibility of reversible causes of heart failure (for example: obstructive sleep apnoea), metabolic stress testing when feasible (stress tests are contra-indicated in patients on inotropes), invasive haemodynamic evaluation, laboratory evaluation of organ function including lungs (pulmonary function tests), renal, liver, and haematologic function. All patients should undergo a psychosocial evaluation to estimate patient psychological status, risk for substance abuse, compliance to treatment and
supporting environment.22,29
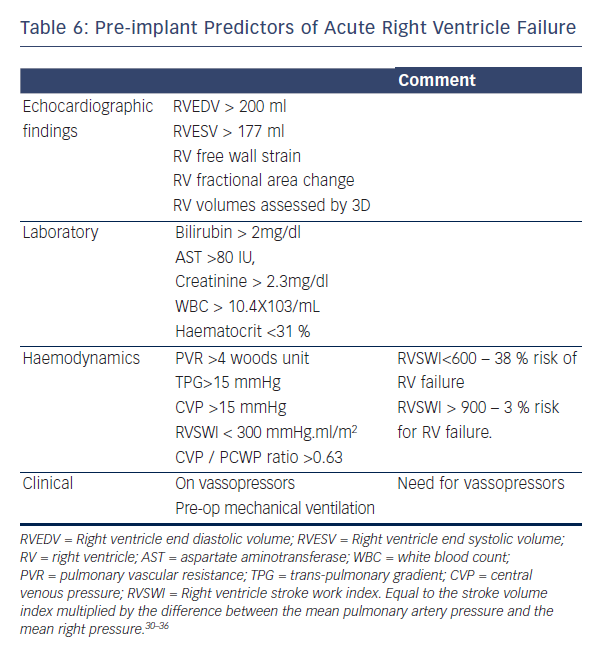
Right ventricular (RV) failure is a leading cause of mortality after LVAD implantation,22 since LVAD optimal function relies on adequate filling of the left ventricle (LVAD preload), which in turn is dependent on RV function. Many studies have tried to predict which patients are at risk for RV failure after LVAD implantation.30–36 Table 6 summarises the major published predictors of post implant RV failure.
The final step in evaluating LVAD candidates is an estimation of a patient’s overall frailty. Frailty was originally a geriatric term defined
as a state of vulnerability to adverse outcomes and decreased physiologic reserve, reflecting the biologic rather than chronologic age.37,38 Frailty is very common among HF patients and adversely affects prognosis39,40. In LVAD patients, frailty is associated with higher post-implant complication rates and mortality.38,41
LVAD Complications
Patients treated with long-term MCS may develop characteristic complications associated with the implantation of the VAD.
VAD Thrombosis
VAD thrombosis, one of the most devastating complications of MCS, is defined as the development of a blood clot within one component of the device, including the inflow cannula, outflow cannula, and the rotor despite anticoagulation and antiplatelet therapy.42 Since 2011, for unknown reasons, there has been a reported abrupt increase in the incidence of HeartMate II VAD thrombosis from 2.2 % before 2011 to 8.4 % in 2013.42 Device thrombosis is also a complication in
HeartWare HVAD devices, reported to occur in 8.1 % of the patients.43 However, unlike the increase in the incidence of HeartMate II VAD thrombosis, the incidence of HeartWare HVAD thrombosis has remained stable since 2008.43,44 With the growth in the number of patients treated with LVAD, the magnitude of this complication will continue to rise if there is no deployable strategy to mitigate the risk of pump thrombosis.45
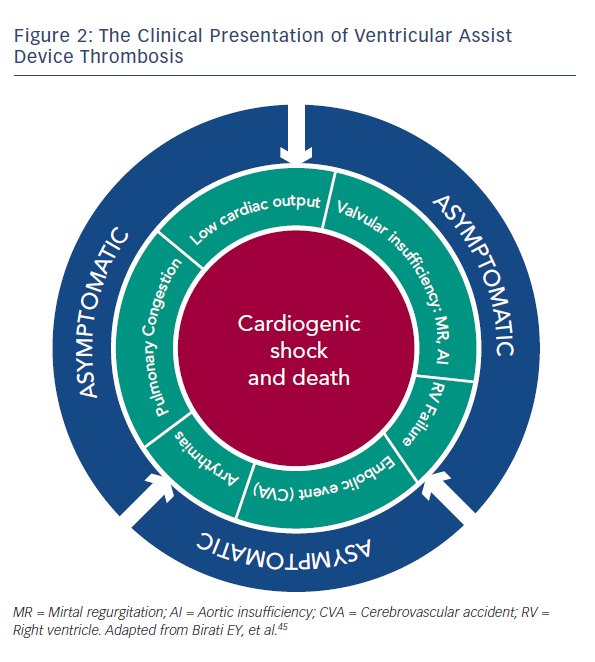
VAD thrombosis has more than one clinical presentation, and can involve a wide spectrum of clinical features, ranging from an asymptomatic patient to one with refractory cardiogenic shock and subsequent death.45 The various clinical presentations are detailed in Figure 2. Even early stages of VAD thrombosis may cause haemolysis that can be identified with elevated lactate dehydrogenase (LDH) levels, indirect bilirubin and plasma free haemoglobin (PFHg) levels.22 Uriel et al.46 reported that an LDH higher than five times normal was 100 % sensitive and 92 % specific for the diagnosis of pump thrombosis46, but our series argue that any value above the normal LDH range can imply VAD thrombosis.45
Patients with a suspected diagnosis of VAD thrombosis should be started on intravenous heparin, unless contraindicated; patients with highly suspected VAD thrombosis should be considered for pump exchange.47 Starling et al.42 showed that the six-month mortality of HeartMate II patients treated with device replacement was similar to the mortality of patients who did not have pump thrombosis.42 In patients with HeartWare HVAD thrombosis it is reasonable to start thrombolysis if the patient is haemodynamically stable and has no contraindications for thrombolytic therapy. However, if the HVAD patient does not improve clinically or suffer from haemodynamic instability, pump replacement should be considered.47
Acute Right Ventricular Failure
Acute RV failure is a frequent complication, occurring in 20–50 % of patients following LVAD implantation, and is associated with increased morbidity and mortality.48–50 Acute RV failure post-LVAD implantation is defined as a need for inotropes longer than 14 days after LVAD implant or the need for temporary RVAD placement after LVAD surgery.48,51
After LVAD placement, there is an abrupt decrease in the left ventricular end-diastolic pressure (LVEDP), followed by a decrease in left pulmonary capillary wedge pressure. In most patients this, in turn, causes a decrease in the pulmonary vascular resistance (PVR), thus decreasing the afterload of the right ventricle.52 However, some patients have a significant shift of the inter-ventricular septum towards the LV secondary to the decrease in the LVEDP and LV size and consequently an increase in RV preload. This shift of the septum adversely affects the function of the RV. High device speeds enhance this inter-ventricular septum shift, and can further deteriorate the RV function. Thus, it is highly recommended to conduct repeat echocardiographic studies during the first days after the implantation and to adjust the pump speed according to the septal movement and the ventricles size. RV failure can result in inadequate filling of the LV. Since the LVAD is preload dependent, patients with acute RV failure may present with cardiogenic shock.
Up to 15 % of patients with acute RV failure will require RVAD implantation.53 These patients suffer from severe RV failure leading to end organ dysfunction and cardiogenic shock refractory to inotropes.53
In an effort to prevent RV failure, some authors advocate for the routine use of phosphodiesterase type 5 inhibition, nitric oxide, or Epoprostenol Sodium (Flolan) therapy in every patient with pre-implant PVR above 3.11
Gastrointestinal Bleeding
Approximately one forth of VAD patients suffer from gastrointestinal bleeding (GI)54; half of the bleeding episodes originate from the upper GI tract. Although angiodysplastic lesions are the predominant cause of bleeding, stress and peptic ulcers are common in this patient population as well.54
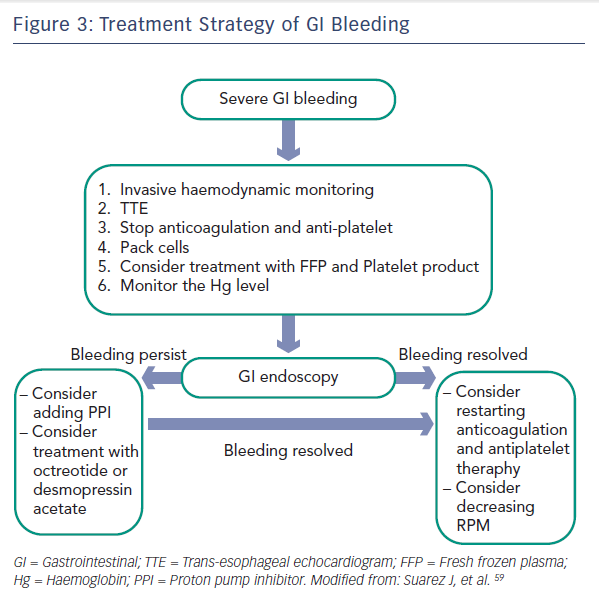
The increased risk of bleeding is associated with several factors. First, similar to the Heyde syndrome of aortic stenosis, LVAD rotors generate high shearing forces leading to degradation of von Willebrand factor and acquired von Willebrand syndrome.55–57 Second, the continuous-flow devices generate low pulse pressures, which may cause GI hypoperfusion leading to formation of angiodysplastic lesions.58 In addition, most VAD patients are treated regularly with anticoagulation and antiplatelet regimens, which further increases the risk of bleeding.54 Figure 3 summarises the recommended treatment strategy of GI bleeding.59
Infection and Sepsis
Infection is a major cause for morbidity and mortality in LVAD
patients. Although the prevalence of VAD associated infections is improving with second and third generation devices, it continues to be a worrisome complication, with 20 % of VAD deaths attributed
to infection.60,61
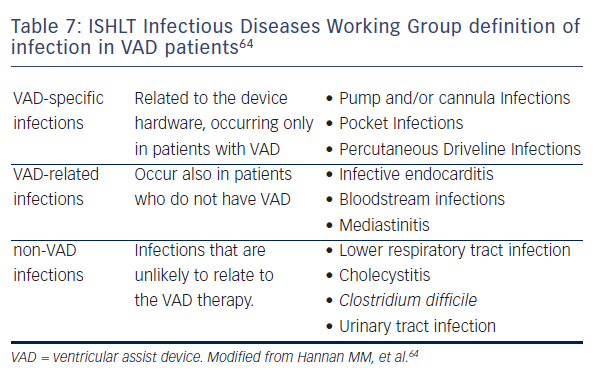
According to the International Society of Heart and Lung Transplantation (ISHLT) data, 87 % of VAD infections are bacterial (primarily Staphylococcus and Pseudomonas species) with the reminder being mostly fungal.62,63 The clinical presentation of VAD infections may be nonspecific and misleading, with symptoms such as lethargy, fatigue, or anorexia, with or without fever or shock.64 Table 7 summarises the ISHLT Infectious Diseases Working Group definition of infection in VAD patients.
Driveline infections are the most prevalent infections in VAD patients and may reflect the presence of a deeper infection of the device hardware (pump, cannula) or the pocket space. Due to the marked variability in the clinical presentation, vigilance is required for an early diagnosis of infection.
The Future of Ventricular Assist Device Therapy
The surge in the prevalence of HF worldwide will result in a substantial rise in the number of patients treated with long-term MCS. The next generation devices are currently being evaluated in clinical trials. These devices are smaller and easier to implant. Moreover, they are designed to have more flexible percutaneous leads, in an effort to decrease the risk of infections.65,66 Future devices will be more physiologic and will be able to automatically accommodate
to the patient’s physical activity and position. In addition, devices in the future will have trans-dermal charging so that the system is totally within the body, which will further decrease the risk of infection, and allow patients to swim and shower with no limitation on daily activities.
With the accumulating experience and improved outcomes, it is likely that the indications for LVAD implantation will expand and include patients with less severe HF. Finally, there is an ongoing interest in treating HF patients with preserved LVEF with durable MCS.67
In conclusion, MCS has emerged as an essential option for advanced HF, with increasing number of patients treated with this modality. These devices significantly improve survival and quality of life in appropriately selected patients. Awareness of the unique complications and clinical presentations is crucial for the long-term management of VAD patient