Heart failure (HF) is a global epidemic which affects about 6 million adults in the US. It is projected that by 2030 the total cost of HF will reach US$70 billion. Despite the development of novel drugs and devices, the mortality burden of HF remains high, with one in three patients dying within 1 year of hospitalisation for HF and 40–50% within 5 years of diagnosis.1
Patients with HF are divided into functional class based on the New York Heart Association (NYHA) classification. NYHA class I–IV refers to the severity of symptoms, with class I patients being asymptomatic with ordinary activity and class II and III patients being symptomatic with ordinary or less than ordinary activity, respectively. HF subjects who develop symptoms at rest or with physical activity can be classified as class IV functional status. This symptomatic classification has been a major entry criterion for the clinical trials that support current HF treatment guidelines.
Outcomes of HF Patients with Reduced Ejection Fraction Based on Functional Classification
Even patients with asymptomatic early stages of HF, without symptoms, have evidence of ongoing adaptive and maladaptive pathways.2 Accordingly, patients with NYHA class I and II still have a relatively high morbidity and mortality burden. In a subanalysis of the Digitalis Investigational Group trial, when 1,863 subjects with NYHA I and II were matched to the same number of subjects with NYHA III and IV, the mortality rates were 34% versus 42%, and all-cause hospitalisations were 66% versus 71%, respectively.3 This shows that patients with a worse functional status have a higher mortality burden and this is behind the reasoning for symptom-driven therapy. However, it also reveals the significant poor outcomes for people who are supposedly less ill, which implies the need for an equally intensively treatment for all patients with HF, irrespective of symptoms, since most of the approved medications have been proven to reduce mortality (and/or morbidity) for the entire range of HF functional status.
It is known that patients with HF, irrespective of ejection fraction and symptomatology, all have increased mortality rates. The most thorough studies investigating the actual cause of death in HF involved ICD or cardiac resynchronisation therapy (CRT). In one study from New Zealand involving almost 400 patients with HF with reduced ejection fraction (HFrEF), the 5-year all-cause mortality rate was 6%, while the relevant sudden arrhythmic death rate only 0.3%.4 In a more inclusive population of HF with reduced, preserved or recovered ejection, with or without implantable devices, and a mean follow-up of 4.5 years, 40% of the population (1,057 patients in the total population) died with 13.9%, 29.6% and 34.4% of the deaths deemed to be due to sudden cardiac death (SCD), worsening of HF or non-cardiovascular causes, respectively.5
In the largest trial to date in HFrEF, the Prospective Comparison of Angiotensin Receptor-Neprilysin inhibitor with Angiotensin Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure study (PARADIGM-HF; n=8442), 81% of deaths had a cardiovascular (CV) aetiology of which 45% were SCD and 26% due to HF.6 SCD comprises a greater proportion of the CV deaths in patients with milder HF symptoms. Clinical trial data have generally demonstrated that neurohumoral antagonism, which is a guideline-directed medical therapy (GDMT) for HF, is able to reduce both the SCD rate and deaths due to the progression of HF.
Reliability of Classification
One of the first limitations to a symptom-driven treatment strategy is the lack of accuracy and reproducibility of symptom classification which is currently based on a doctor’s consultation. When two cardiologists were asked to characterise the NYHA class of patients with mild to moderate symptoms, there was a low concordance of only 54–56%.7,8 Others have suggested that we should use self-reported NYHA classification, allowing the patient to determine their classification, but a study reported a poor correlation compared with classifications from physicians.9 Implementation of more objective measures of activity tolerance, such as the 6-minute walk test (6MWT), or cardiopulmonary exercise test – which is currently the gold standard – could address this issue but can be difficult to obtain routinely.
A large meta-analysis has demonstrated a large heterogeneity between 6MWT distance and NYHA class. It also showed that the NYHA classification system was better able to distinguish functional capacity between class III and IV than between I and II.10 In another study with 145 subjects, only a 42% agreement was found between the NYHA classification and the more advanced and accurate VO2 max levels in cardiopulmonary exercise testing, while in a retrospective analysis from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, no clear association was found.11,12 Taking a patient’s medical history in a more standardised fashion or using a specific questionnaire might help improve accuracy and reliability, but it cannot be ignored that the perception of symptoms is influenced by multiple factors that are not necessarily related to HF. Therefore, newer technology in the form of novel sensors and activity monitors, which provide real-time continuous activity information, might be a better measure, but they need to be validated in well-designed clinical trials.
Guideline Recommendations
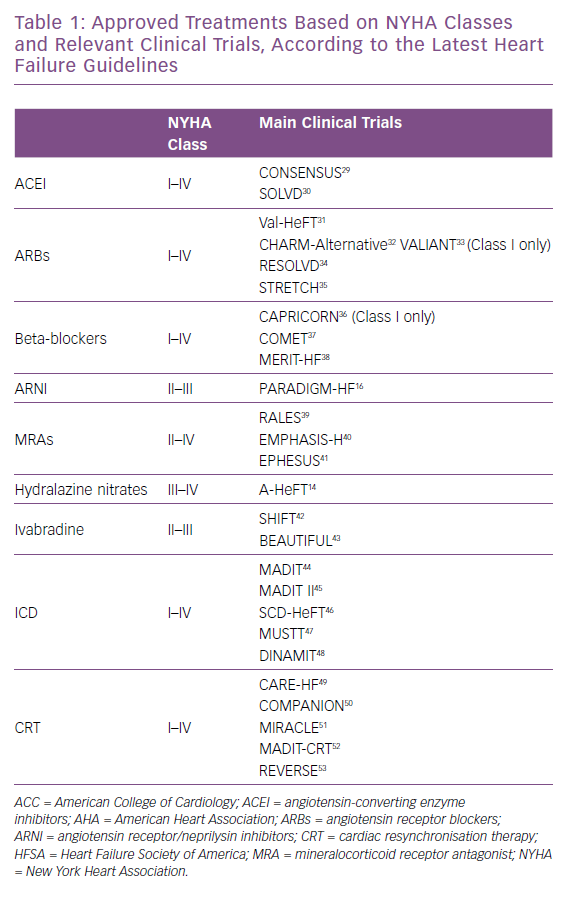
The latest American College of Cardiology/American Heart Association HF guidelines suggest that angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARBs), beta-blockers, ICDs and cardiac resynchronisation therapy (CRT) play a substantial role in the management of people with HF with structural heart disease with prior or current symptoms; stage C as defined by the inclusion criteria of the trials that established their efficacy.13 It should be noted that there is scant evidence regarding the use of beta-blockers and ARBs in patients with NYHA class I HF.
In the African-American Heart Failure Trial (A-HeFT) the combination of isosorbide dinitrate and hydralazine was only tested in patients with NYHA class III/IV, showing a significant reduction in the composite endpoint of all-cause mortality or first HF hospitalisation.14 Mineralocorticoid receptor antagonists (MRAs) are indicated for NYHA class II-IV, and angiotensin receptor/neprilysin inhibitors (ARNIs) for only classes II to III, despite the evidence showing beneficial pathophysiological effects of the former in NYHA class I patients and a mortality benefit better than any other evidence-based therapy available today for the latter (Table 1).15,16 In the current era of complicated healthcare logistics, and while awaiting the results on efficacy and safety of sacubitril/valsartan from the Entresto™ (LCZ696) In Advanced Heart Failure (LIFE Study), more studies into cost-effectiveness of these treatments in populations with certain characteristics should be initiated to justify the use of this or any other expensive therapeutic strategy.
Undertreatment of Heart Failure
Beyond the fallibility of the symptomatic assessment of HF, it is clear that symptomatic HF patients are undertreated. Providers may falsely believe that patients with milder symptoms have low morbidity and mortality and that patients with advanced disease may be ‘beyond help’.17
Recent data from the Change the Management of Patients with Heart Failure (CHAMP-HF) trial reveal the extent of undertreatment of HF. In real life conditions, only 1% of patients were receiving all GDMT at target doses, while 27%, 33% and 67% were not prescribed with ACEI/ARB/ARNI, beta-blocker or MRA respectively.18 It is known that the underuse of indicated medication classes, as well as the lack of uptitration of these agents, leads to worse outcomes.19,20
GDMT and advanced therapies have proven beneficial effects on a cellular and myocardial level, even in subjects with advanced HF.21,22 However, since rejuvenative therapies, such as stem cells, have failed to offer sustained long-term benefits, the current therapeutic options are able to modify the underlying pathophysiology only in the absence of scar and fibrosis.
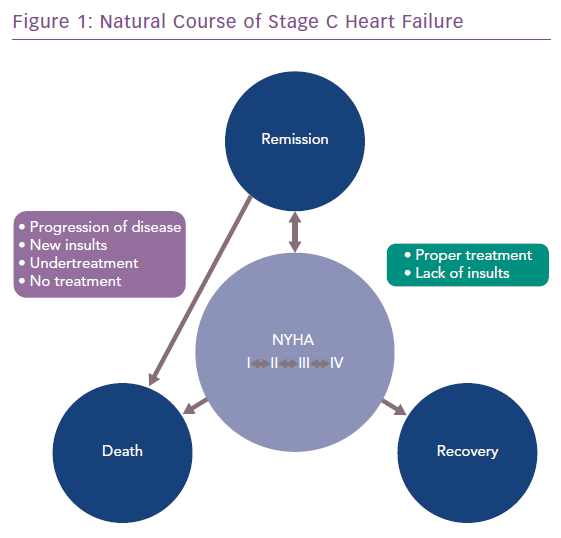
Some people with dysfunctional and viable myocardium amenable to reverse remodelling. A small study of people with ischaemic HF showed that 19% and 60% of patients with NYHA I or II, respectively, had dysfunctional but viable myocardium, based on cardiac MRI with gadolinium imaging.23 Proper medical management and control of risk factors that alter contractility, preload and/or afterload, could potentially delay progression or even cause regression of the disease and lead to remission or recovery (Figure 1).
Based on the findings of CHAMP-HF, NYHA class IV was associated with less than optimal GDMT, potentially due to the belief of physicians that treatment was futile, along with higher levels of medication intolerance. However, all approved HF medications, except for ARNI, have reduced morbidity and mortality in this HF patient population. Additionally, some of these patients are candidates for mechanical circulatory support (MCS) devices to further improve the quality and quantity of life. A minority of this MCS population may achieve remission of their HF, allowing later explantation of the device.24
There are two unanswered questions regarding this strategy: how to recognise viable and dysfunctional myocardium, which has been challenging even when using cardiac MRI, considered to be the most sensitive and reliable method; and how to differentiate the myocardium from being in real recovery or temporary remission. There is a need for further research into these issues which could be assisted with the use of novel emerging biomarkers.
Modern HF therapeutic strategies prolong survival by inducing reverse remodelling of the ventricle. It has been uncertain whether GDMT should be continued in patients with significant improvement in left ventricular ejection fraction. In these patients, it could be argued that we would be able to discontinue most of their HF mediations, or even treat them based on their minimal symptoms. Other mostly retrospective studies suggested medical therapy be continued in this population, with the exception of people with peripartum cardiomyopathy.25,26 However, the recently published TRED-HF provides the first evidence from a randomised clinical trial, suggesting that even in the absence of symptoms in this population, the residual activity of pathophysiological mechanisms require the continuation of GDMT to prevent relapse.27
HF is a chronic disease with a similar, if not worse, prognosis than other serious and life-threatening conditions, such as cancer or chronic kidney disease. Patients with HF are treated by multiple healthcare professionals, including cardiologists, hospital doctors and generalists. This is entirely different from care for the aforementioned diseases, which are treated only by specialists, regardless of the stage of the disease or the intensity of reported symptoms. This is of particular importance when inpatient management is involved. HF hospitalisation is a significant event in the physical course of HF, being an extremely negative prognostic factor. At the same time, it is a unique opportunity to initiate and establish an effective therapeutic plan. Only patients with more severe disease that does not respond to treatment are seen by cardiologists, which confirms the misconception that HF has a ‘benign’ disease course, especially among subjects whose symptoms quickly improve.28
Guideline treatment recommendations are based on the enrollment criteria of the relevant clinical research. Current trends in the design of HF clinical trials should expand the evidence base to include a broader range of HF patients. Inclusion criteria and endpoints using objective parameters, such as biomarkers and imaging indices, rely less on subjective symptoms. Enrolling older patients with significant kidney insufficiency, who in the past have been excluded, is a move in the right direction. Obtaining more objective measures is especially true in the trials being conducted for the treatment of HFpEF, for which we still do not have a satisfactory evidence-based treatment strategy. This could have a significant effect on daily practice, by making beneficial medications available to a larger number of patients, faster than in the past.
The problem of undertreating HF persists despite the availability of multiple potent therapeutic strategies. However, for the first time after many years, the scientific community has intensified the discussion with supporting evidence. We are transitioning slowly to a new paradigm for treating HF aggressively at earlier stages and rigorously even at the more advanced stages, based on more objective parameters than symptoms alone. All patients should be treated intensively, irrespective of their functional status, to prevent disease progression and maximise the likelihood of HF remission and/or myocardial recovery. Ongoing and future clinical trials will provide the data necessary to advance this treatment strategy among healthcare professionals and patients as a significant culture change.