Neprilysin (NEP) was more or less unrecognised by the cardiovascular community until 2014 when the Prospective Comparison of ARNi With ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study reported impressive clinical benefits resulting from the combination of an angiotensin receptor blocker (ARB) with a neprilysin inhibitor (angiotensin receptor neprilysin inhibitor [ARNi]) over angiotensin-converting enzyme (ACE) inhibition in heart failure with reduced ejection fraction (HFrEF).1 The study was stopped prematurely due to the positive results. The first ARNi holds a class IB recommendation for stable systolic heart failure (HF) with a left ventricular ejection fraction (LVEF) of ≤35% in individuals who remain symptomatic on recommended doses of ACE inhibitors/ARBs and have elevated natriuretic peptide levels according to current treatment guidelines.2
More recent data suggest that an extended spectrum of HFrEF patients, including those with moderately-to-mildly reduced LVEF, might also benefit from the therapy. Although the Efficacy and Safety of LCZ696 Compared to Valsartan on Morbidity and Mortality in Heart Failure Patients with Preserved Ejection Fraction (PARAGON-HF) study, which tested ARNi in HF patients with LVEF 45% or higher, missed its primary endpoint, subgroup analysis implied it had a beneficial effect in patients with lower LVEF and, interestingly, women.3
The Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on NT-proBNP in Patients Stabilized from an Acute Heart Failure Episode (PIONEER-HF) and Comparison of Pre- and Post-discharge Initiation of LCZ696 Therapy in HFrEF Patients After an Acute Decompensation Event (TRANSITION) studies imply that initiation in decompensated HF patients is safe and effective.4,5 The new guidelines of the European Society of Cardiology are egarely awaited, especially concerning a potential upvaluation of ARNi within the therapeutic algorithm for treating HF.
The success of this new therapeutic strategy has encouraged research into the role of NEP in HF, the regulation NEP and the mechanism of action of NEP inhibition. Understanding NEP regulation would probably enable the assessment of an individual patient’s NEP status, which is potentially useful for risk stratification and therapy guidance, and may reveal previously unrecognised pathomechanisms of HF, paving the way for novel therapies.
Biology of Neprilysin
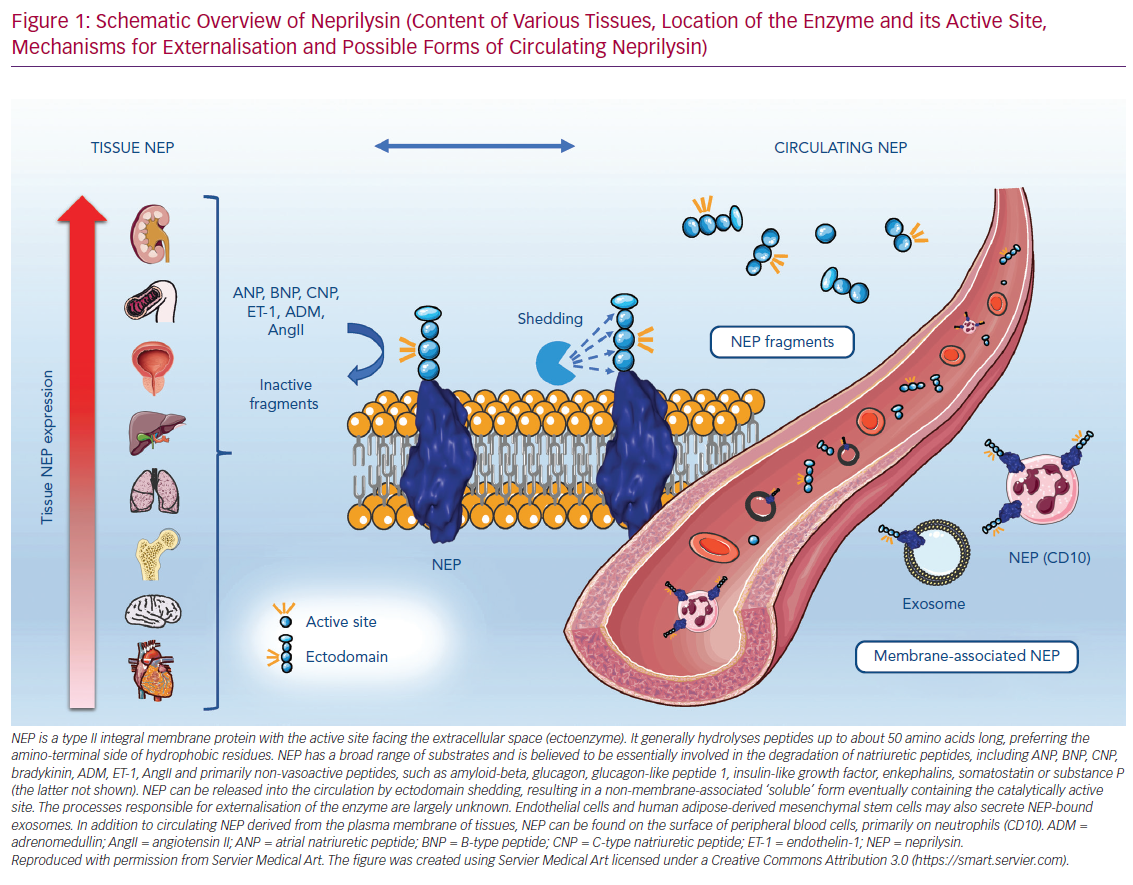
The human genome covers about 686 putative peptidases that regulate the breakdown of bioactive peptides involved in key biological processes.6 The zinc-dependent metallopeptidases form a large group of enzymes that includes NEP, ACE, carboxypeptidases and collagenases, among others.7 Human endopeptidase NEP shows a highly conserved sequence homology with other species, including rodents and pigs.8 It consists of 749 amino acid residues and, as a type II integral membrane protein, is located in the plasma membrane with the active site facing the extracellular space. This multisubstrate-metabolizing enzyme generally hydrolyses peptides of up to about 50 amino acids long, preferring the amino-terminal side of hydrophobic residues; it sometimes acts more efficiently as a dipeptidyl carboxypeptidase than a true endopeptidase.7 Given the diverse range of substrates, NEP has been discovered in many different enzymatic contexts in the past, resulting in it having multiple names, including membrane metalloendopeptidase EC 3.4.24.11, neutral endopeptidase 24.11, endoprotease 24.11, common acute lymphoblastic leukaemia antigen (CALLA), neutrophil antigen cluster differentiation antigen 10 (CD10) and enkephalinase.
NEP is believed to be involved in the degradation of natriuretic peptides, including atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), adrenomedullin, endothelin-1 and angiotensin II, as well as primarily non-vasoactive peptides, such as glucagon, glucagon-like peptide, enkephalins and somatostatin.9 The specificity of NEP is determined by the length and subsites of the substrate and the dissociation rate of the two metabolites formed on release from the active site.7 Although the affinity of NEP towards its putative substrates may be predicted to some degree, and turnover rates have been investigated for some peptides, its singular functional relevance is difficult to forecast in vivo. Moreover, NEP is a fairly ubiquitous enzyme. It is found in the highest concentrations within the kidneys, followed by the gastrointestinal tract, liver, male genital organs, lungs and adipose tissue, and has also been detected in the brain and heart.10 NEP is expressed on a variety of cells, including epithelial cells, endothelial cells, fibroblasts, smooth muscle cells and cardiac myocytes.11
Neprilysin Inhibition by Sacubitril/Valsartan
PARADIGM-HF was the first study definitively proving that NEP inhibition is beneficial in HFrEF, after decades of research investigating the effects of different NEP inhibitors in animal models, as well as human HF and hypertension. NEP inhibition consistently resulted in an increase in circulating natriuretic peptides (ANP and BNP), and was related to natriuretic peptide effects via elevated cyclic guanosine monophosphate concentrations, vasodilation and natriuresis.12–15 The enhanced activity of bradykinin, another substrate of NEP, might contribute to the beneficial effects, but this effect is still to be investigated in combination with an ARB/ARNi.16 NEP inhibition was regarded to primarily affect the natriuretic peptide system, whereas simultaneously activated the renin–angiotensin system (RAS). The inhibition of NEP was subsequently discussed as a beneficial cardiovascular target alongside the use of ACE inhibitors, which again alleviate the non-desirable activation of RAS. Encouraging preclinical data have led to the NEP inhibitor omapatrilat being tested in HF.
In the Inhibition of Metalloprotease by omapatrilat in a Randomized Exercise Symptoms Study with heart failure (IMPRESS), omapatrilat reduced the composite endpoint compared to lisinopril in symptomatic HF patients already on an ACE inhibitor.17 However, when compared to enalapril in the Phase III Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE), omapatrilat failed to show a reduction in the primary endpoint in symptomatic HF patients already on an ACE inhibitor.18 The increased risk of severe angioedema, probably due to excess bradykinin, led to the cessation of vasopetidase, that is, a NEP inhibitor plus an ACE inhibitor, development. This problem could be overcome by combining a NEP inhibitor with ARB instead of an ACE inhibitor in ARNi.
In healthy controls, the administration of sacubitril/valsartan results in dose-dependent increases in ANP, cyclic guanosine monophosphate, renin concentration and activity, as well as angiotensin II.19 An increase in renin and angiotensin II is also observed in HFrEF patients shortly after therapy initiation.20 Notably, angiotensin II actions are assumedly sufficiently blocked by the ARB component. Moreover, NEP inhibition in HFrEF leads to increased circulating levels of NEP substrates, such as ANP, glucagon-like peptide 1 and substance-P.21 Beyond these changes in plasma levels of bioactive peptides, which might be based on direct or indirect effects of NEP inhibition, more clinically apparent features of ARNi therapy have been described. ARNi results in a long-term improvement in echocardiographic parameters, might improve functional mitral regurgitation, exerts nephroprotective effects and improves glycaemic control.22–25 The precise mechanisms underlying these clinical features and, most interestingly, how NEP inhibition reduces cardiovascular mortality and HF hospitalisation remain elusive. Understanding NEP regulation in HF conditions and the effects during treatment with NEP inhibitors would grant profound insight into the pathophysiology of HFrEF, which is necessary for the progression of HF therapy.
Neprilysin as a Biomarker
Given the convincing clinical benefit of NEP inhibition in HFrEF, it should be assumed that individual NEP regulation is associated with disease severity, therapy response, the occurrence of side-effects and outcome. The precise determination of NEP regulation could therefore be of great importance, especially as the number of patients treated with the drug is growing rapidly. Biomarker-guided strategies might enable the monitoring and optimisation of therapies in individuals. NEP inhibition is excitingly successful in HF, but also seems to exert beneficial effects on other conditions, such as chronic kidney disease (CKD) and diabetes.1,23 Understanding how NEP is regulated and the mechanisms involved in NEP inhibition might cast light on the pathomechanisms of HF and systemic disease, possibly pointing to novel therapeutic targets.
To date, the clinical utility of measuring circulating NEP, that is, NEP concentrations or activity or neutrophil NEP expression, is hypothetical. Establishing reliable analytical methods for determining NEP concentrations and actions are a prerequisite. Yet, the determination of NEP – or at least its concentration – seems to be challenging.
Circulating Neprilysin
Circulating plasma biomarkers represent the most convenient and feasible approach to addressing this issue. NEP, like many other membrane-bound metalloproteases, can be released from the cell surface by ectodomain shedding into the extracellular milieu, resulting in a non-membrane-associated ‘soluble’ form containing the catalytically active site. The processes responsible for the externalisation of the enzyme are largely unknown. A disintegrin and metalloprotease 17 (ADAM-17) plays a role in NEP release.26 Endothelial cells and human adipose-derived mesenchymal stem cells may also secrete NEP-bound exosomes.26,27 NEP is not only ubiquitously expressed in various tissues but can also be found on peripheral blood cells (called CD10 here), primarily on neutrophils. Figure 1 illustrates the relationship between tissue NEP and its mebrane and non-membrane associated circulating forms. The following section summarises data on the possible use of different forms of circulating NEP as a biomarker, with a focus on cardiovascular diseases.
Non-membrane-associated Serum/Plasma Neprilysin Concentrations
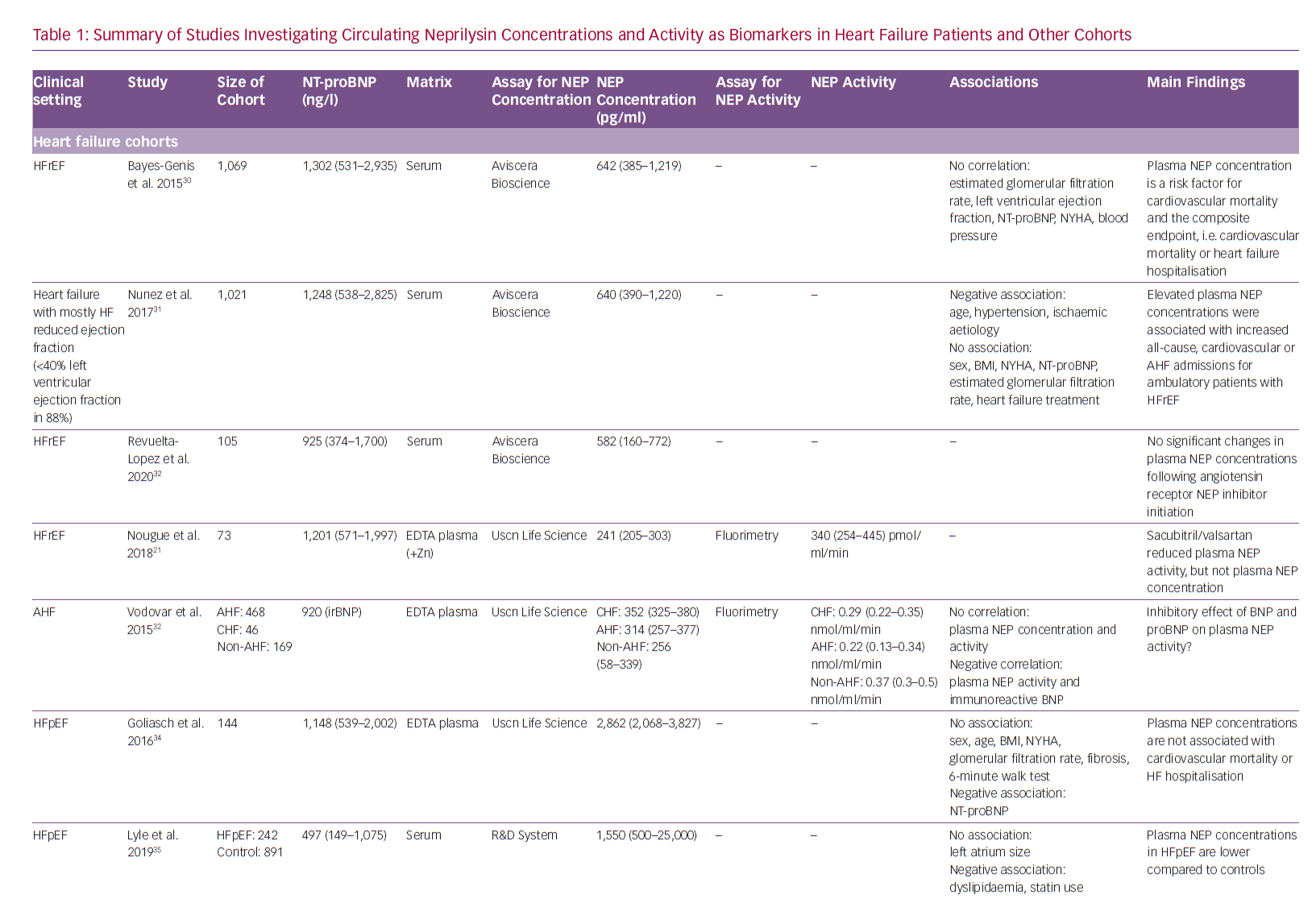
Non-membrane-associated NEP has been detected in serum/plasma in addition to urine, cerebrospinal and synovial fluid.28,29 Circulating NEP concentrations have been evaluated in several types of HF and some other cohorts. Table 1 summarises the studies investigating circulating NEP in distinct diseases. Although there is a reasonable rationale as to why serum/plasma NEP might be related to disease states and prognosis in various cohorts, NEP levels were rarely observed to be associated with disease severity and outcomes.
To date, seven studies have been conducted in HF cohorts: four included stable HFrEF, one acute decompensated HF and two HF with preserved ejection fraction (HFpEF). The first and largest study, which included 1,069 HFrEF patients reported a significant direct association between serum NEP concentrations and cardiovascular mortality with NEP as a risk factor, but no correlation between serum NEP and HF disease severity, as assessed by left ventricular ejection fraction, New York Heart Association class or N-terminal pro hormone BNP (NT-proBNP) levels.30 The same group reported data in an equally large, mostly HFrEF population, where elevated circulating NEP concentrations were associated with increased all-cause and HF hospitalisations.31 Again, circulating NEP concentrations were not related to HF severity or treatment.31 Circulating NEP concentrations were lower in decompensated HF than stable HF in another study.32 The initiation of ARNi therapy in HFrEF did not alter plasma NEP concentrations.21,33 For HFpEF, plasma NEP concentrations could not be associated with functional status or outcome.34,35
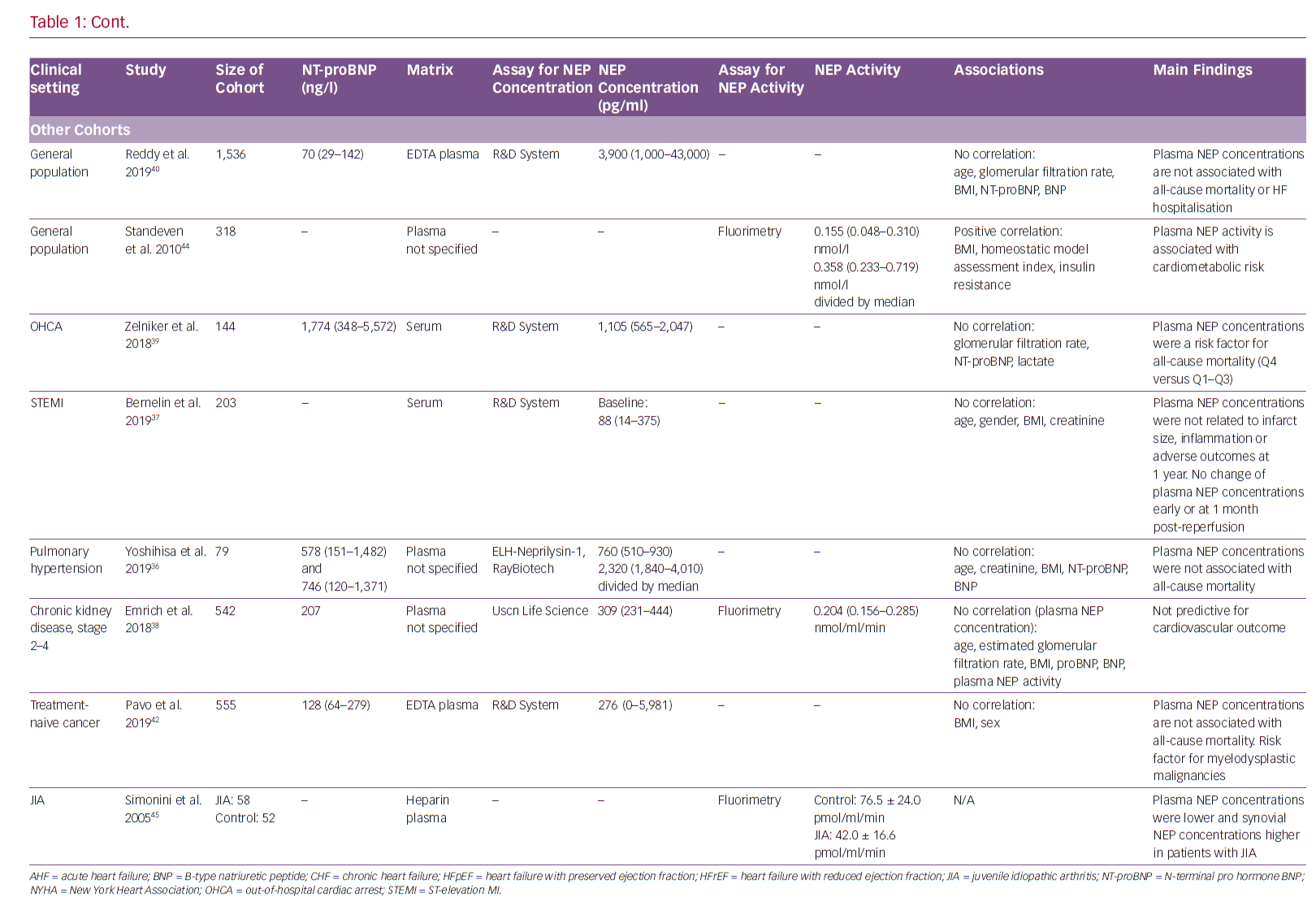
With regard to non-HF cohorts, plasma NEP concentrations did not correlate with NT-proBNP, haemodynamic parameters or outcome in pulmonary hypertension.36 In ST-elevation MI patients, plasma NEP levels did not change significantly in the early phase or 1 month after reperfusion and there was no association with infarct size, inflammation or outcome at 1 year.37 For patients with CKD, plasma NEP concentrations were not associated with hospitalisation for HF or atherosclerotic cardiovascular events.38 In contrast to these neutral results, elevated plasma NEP concentrations were associated with higher all-cause mortality in out-of-hospital cardiac arrest patients, albeit NEP levels were not related to lactate.39 In a relatively large community-based population study including 1,536 individuals, circulating NEP concentrations were not associated with natriuretic peptides or outcomes; however, a link between low NEP levels and an adverse cardiovascular risk profile was suggested.40 NEP is thought to play a major role in cancer development and progression.41 Nevertheless, there were no differences in plasma NEP concentrations for distinct tumour entities or stages, whereas it seemed to be a risk factor for mortality in myelodysplastic disease.42
It seems that plasma NEP concentrations are poor predictors of outcomes in HF and other diseases and might not be ideal biomarkers. The major limitation in interpreting these data is the lack of consistency between the different immunoassays used, as also there are no data available on pre-analytics, constitution of NEP fragments and corresponding antibodies.16
Non-membrane-associated Circulating Neprilysin Activity
Circulating NEP retains some of its catalytic activity, as NEP activity is detectable in plasma. It has been suggested that plasma NEP activity might correlate with circulating concentrations of NEP fragments.43 However, the study investigating chronic and decompensated acute HF patients found no correlation between plasma NEP concentrations and activity.32 Plasma NEP concentrations were not related to activity in CKD patients either.38 In contrast, NEP inhibition in HFrEF patients receiving ARNi therapy resulted in decreased plasma NEP activity.21 In CKD, higher plasma NEP activity, but not concentration, was associated with a lower incidence of hospitalisation for HF or cardiovascular events.38 Plasma NEP activity has also been implicated in other non-HF contexts. Plasma NEP activity increased with indices of metabolic syndrome, such as insulin resistance, homeostatic model assessment index and BMI, in 318 otherwise-healthy adults in a previously published study.44 In patients with juvenile idiopathic arthritis, plasma NEP activity was lower than in control subjects.45
Membrane-associated and circulating NEP exhibit similar affinity for inhibitors, optimal pH and Km (Michaelis constant) range, although the maximum reaction rate of circulating NEP is lower than its membrane-associated equivalent; therefore, in vivo NEP activity is believed to be predominantly tissue-based.46,47 In vivo measurement of tissue NEP activity has not been established and is not feasible. Circulating NEP activity might better reflect tissue NEP regulation, suggesting its superior ability as a biomarker when compared to circulating NEP concentrations. However, the contribution of different tissues to systemic NEP actions remains unclear. As NEP has a wide anatomical distribution and diverse biological functions, it can be assumed that its regulation is complex and that altered levels might be found in many disease types. Cardiac disease-specific changes might be difficult to outline.
Functional Membrane Neprilysin Expression on Neutrophils
As discussed, there are currently no data on the relationship between tissue NEP activity and plasma NEP concentrations or activity. Based on the poor association of plasma NEP measures with clinically relevant parameters and the unknown mechanisms involved in enzyme release, it may be assumed that tissue NEP is not related to plasma NEP measures. Consequently, the assessment of functional, membrane-associated NEP might be more reasonable. As discussed, NEP is ubiquitously expressed in various tissues and on peripheral blood cells (here called CD10), primarily on neutrophils. NEP on neutrophils may play a role in chemotaxis and neutrophil responsiveness to inflammatory stimuli.48,49 It has been shown to modulate neutrophil function by regulating concentrations of ANP and BNP.50
NEP expression on neutrophils has rarely been investigated, and in different settings immunoreactive NEP expression on neutrophils was upregulated on the addition of stimulating agents and neutrophil NEP fluorescence was elevated in the early phase of acute MI, returning to normal values 7 days after the event.50,51 Patients with severe infections showed a marked decrease in neutrophil NEP expression; septic patients were characterised by decreased neutrophil CD10 expression capacity.51,52 NEP null mice appeared developmentally normal but were sensitive to endotoxic shock.53 A study of 99 patients with HFrEF confirmed the abundant expression of NEP on granulocytes.53 NEP fluorescence intensities were inversely correlated with HF disease severity, that is, NT-proBNP levels and New York Heart Association class, whereas higher expression levels seemed to be associated with better overall survival.54
In summary, against the background of beneficial effects of NEP inhibition, the association between increased NEP expression and better disease state seems counterintuitive. Interesingly, data from the PARADIGM study imply that more stable patients, who might be characterised by higher NEP expression, profit more from NEP inhibition than those with more advanced disease.55 Within this context, the role of neutrophil NEP expression and its possible relationship with proinflammatory state remains to be understood. Whether neutrophil NEP expression could be a surrogate for systemic tissue NEP activity or may be associated with HFrEF outcomes through its reflection of inflammatory predisposition needs to be investigated in future studies.
Urinary Neprilysin and Neprilysin in Cerebrospinal Fluid
For the sake of completeness on non-tissue-based NEP measurements, determination of NEP in urine and cerebrospinal fluid will be discussed shortly. NEP expression is highest in the kidneys, yet the main location of the enzyme is within the brush border of the proximal tubule on the luminal site. Urinary NEP concentrations are increased in critically ill patients with acute kidney injury and in patients with diabetes, especially those with microalbuminuria, suggesting that urinary NEP might be an indicator of acute and chronic kidney injury.56,57 However, urinary concentrations are not correlated with plasma concentrations, resulting in questions about the contribution of kidney NEP regulation to plasma NEP levels.56
NEP in cerebrospinal fluid has been investigated in Alzheimer’s disease. This disease is characterised by amyloid-beta deposits resulting from a disturbed balance between amyloid-beta biosynthesis and clearance.58 NEP is a member of the amyloid degrading enzyme family and seems to be the major enzyme responsible for amyloid-beta breakdown in the brain.59 A meta-analysis showed that NEP expression and activity are reduced in the cortex of individuals with Alzheimer’s disease.60 Accordingly, these patients have lower NEP activity levels in the cerebrospinal fluid compared to controls.61 NEP upregulation could therefore be a valuable therapeutic target in combatting Alzheimer’s disease. However, unselective upregulation of NEP might have deleterious effects on the cardiovascular system. The long-term effects of NEP inhibition by sacubitril/valsartan, with the potential promotion of amyloid accumulation in the brain, need to be fully assessed.
Future Directions
The utility of non-membrane-associated circulating NEP concentrations and activity as biomarkers of NEP regulation are limited, as the physiological actions of NEP are localised to the tissues. Further studies are needed to investigate tissue NEP regulation in HF and establish a relationship with HF disease states and outcomes. Membrane-associated neutrophil NEP expression may be a surrogate for tissue NEP regulation or proinflammatory state.