Heart Failure (HF) constitutes a major global health problem, evidenced by substantial morbidity and mortality, requiring enormous healthcare-related expenditure. HF is associated with high symptomatic burden, and with a relentless and progressive clinical course towards end-stage disease. A large body of epidemiological data suggests that the prognosis in HF is as poor as in advanced cancer.1 Survival after first hospitalisation for HF is very poor, and less than 50 % of patients are alive after 5 years.2,3 By contrast, cardiac transplantation has very favorable 1- and 10-year survival rates of approximately 90 % and 50 %, respectively, but is restricted to an extremely select group of patients. Medical therapy therefore remains the treatment of choice for most patients with HF. HF is divided clinically according to left ventricular ejection fraction (LVEF) into reduced (<40 %), preserved (>50 %) and the newlyintroduced category of intermediate or “midrange” ejection fraction (40–49 %).4
A key feature of chronic HF is the sustained activation of endogenous neurohormonal systems in response to impaired cardiac pumping and/or filling properties. It is widely believed that neurohormonal systems are essential survival and “injury response” mechanisms that have evolved over thousands of years in order to cope with hostile environments and variable climates.5,6 Neurohormonal systems provide survival benefits through actions such as water and salt conservation or vasoconstriction (for example minimising the impact of haemorrhage). In addition, many neurohormonal systems are essential for normal embryonic development.7,8
While these neurohormonal systems may have compensatory haemodynamic effects in the initial stages of HF, chronic stimulation and dysregulation occurs that exerts profound deleterious actions on a broad range of cardiovascular (CV) tissues. When LVEF is in the midrange or preserved categories, guidelines require additional evidence of elevated natriuretic peptide levels for a diagnosis of HF.4
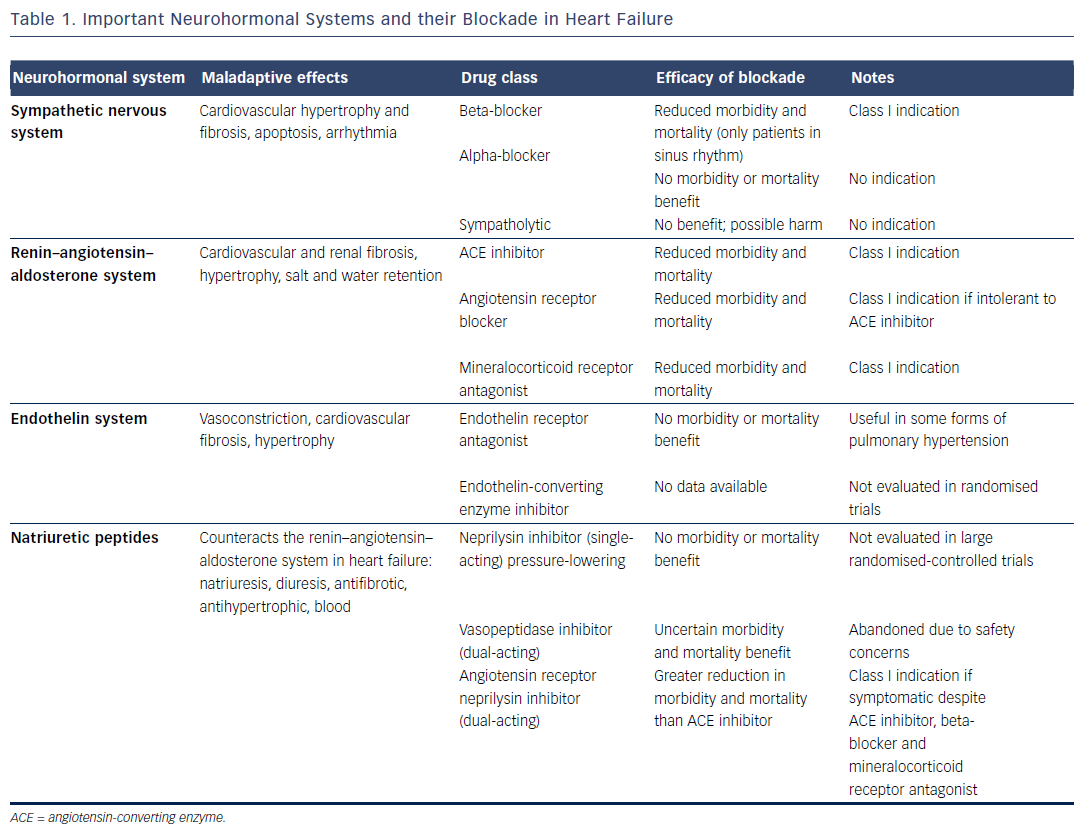
Based on the above considerations, and following scrutiny of randomised clinical trials (RCTs), pharmacological agents that counteract adverse neurohormonal actions have been introduced into clinical practice over the past three decades. The sympathetic nervous system (SNS) and the renin–angiotensin–aldosterone system (RAAS) are major neurohormonal systems that exert potentially maladaptive actions in HF.9 In patients with HF with reduced ejection fraction (HFrEF) in sinus rhythm, pharmacological blockade of these systems has been shown to markedly reduce mortality and morbidity (see Table 1).4,10–15 As yet, no medical therapy has been shown to improve the prognosis of patients with HF with preserved ejection fraction (HFpEF), despite evidence that both systolic and diastolic dysfunction affect the sympatho–vagal balance.16
This article provides an assessment of the major neurohormonal systems and their therapeutic blockade in patients with chronic HF.
The Sympathetic Nervous System and Pharmacological Blockade
Activation of the SNS increases stroke volume and induces peripheral vasoconstriction in order to maintain arterial perfusion pressure.
The interface between the sympathetic nerve fibres and the CV system is formed by the adrenergic receptors. In HF, sustained sympathetic stimulation through elevated catecholamine levels (noradrenaline and others) leads to reductions in cardiac beta-1- adrenergic receptor density and function over time, contributing to disease progression.17–20 Initially thought to be contraindicated in HF, beta-adrenergic receptor antagonists (beta-blockers) represent a cornerstone of the current medical management of HF based on a well-documented reduction in clinical event rates.4,21,22
The beneficial actions of beta-blockers are believed to occur through mechanisms including reduced heart rate and myocardial oxygen demand, to reduce the incidence of arrhythmia and sudden cardiac death, and to provide protection from ischaemia. These adaptations to the pathophysiology of HF and their resultant effects on autonomic and neurohormonal balance translate into tangible patient benefits: in HFrEF with sinus rhythm, beta-blockers lead to a 24 % relative reduction (4 % absolute reduction) in all-cause mortality, and a similar reduction in hospital admissions.15 The beta-blockers with proven survival benefit in HF recommended by the European Society of Cardiology and Heart Failure Association guidelines are bisoprolol, metoprolol, carvedilol and nebivolol.4 While bisoprolol and metoprolol are highly selective for the beta- 1-adrenergic receptor, carvedilol possesses broader substrate specifities, having alpha-adrenergic and proposed pleiotropic and antioxidant properties.23
Recent data suggest that the survival benefit of beta-blockers in patients with HFrEF does not extend to those with concomitant atrial fibrillation (AF).21,24 The role of the autonomic nervous system in the (patho)physiology of AF is complex and is related to the modulation of both sympathetic and parasympathetic responses.25 When AF develops in patients with HFrEF, central sympathetic activity is augmented, but the appropriate sympathetic response to exercise is diminished.26,27 These observations raise the possibility that lack of beta-blocker efficacy in AF may be related to differences in autonomic function (and, consequently, the neurohormonal axis), a likelihood supported by the observation that heart rate is associated with mortality in HFrEF with sinus rhythm, but not in HFrEF with AF.28
Blockade of other adrenergic signalling pathways, such as alphaadrenergic receptors, has been ineffective in HF. In patients with hypertension in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) the alpha receptor-blocker doxazosin doubled the incidence of HF, although overall mortality was similar.29 Some sympatholytics, such as hydralazine and clonidine, have been used in resistant hypertension.30 In African Americans hydralazine has been reported to be of benefit.30 Other centrally-acting sympatholytics have shown signs of harm in HF.31 Non-pharmacological strategies to block the SNS in HF, such as catheter-based renal sympathetic denervation and vagal nerve stimulation, are currently undergoing evaluation in clinical trials.32–34
The Renin–Angiotensin–Aldosterone System and Pharmacological Blockade
The RAAS is a vastly complex neurohormonal system including the protagonist hormones angiotensin-II and aldosterone. Angiotensin- II and aldosterone mediate a range of maladaptive actions upon chronic activation, including renal water and sodium retention, peripheral vasoconstriction leading to hypertension, and cellular effects such as hypertrophy and fibrosis of the heart, kidney and vasculature. The first RAAS blockers were introduced in the late 1980s, with angiotensin-converting enzyme (ACE) inhibitor use being supported by a number of clinical trials in HFrEF that demonstrated substantial reductions in mortality and morbidity.35 Angiotensin receptor blockers (ARBs) are recommended only as an alternative in patients intolerant of an ACE inhibitor.4
Renin is located upstream of ACE in the pathway and constitutes a rate-limiting step in the generation of biologically-active angiotensin-II. Therapeutic inhibition of this first specific step in the cascade using direct renin inhibitors was thought to potentially offer therapeutic advantages over ACE inhibition.36 The recent Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE) trial, however, showed that the addition of aliskiren to enalapril increased adverse events without providing any clinical benefit. In addition, statistical non-inferiority could not be demonstrated for monotherapy with aliskiren as compared with enalapril.37 A number of trials have investigated the potential utility of blocking RAAS at multiple levels – not only in HF but also in other CV diseases – and have failed to demonstrate a consistent benefit for dual-acting RAAS blockade.38–42 It therefore seems that adequate RAAS blockade with a single agent (i.e. the maximum tolerated dose of an ACE inhibitor) ensures adequate blockade of angiotensin-II signalling that cannot be enhanced by the addition of an ARB or a direct renin inhibitor.
Beyond angiotensin-II, the mineralocorticoid hormone aldosterone exerts potent cardiorenal fibrosis and hypertrophy and often escapes RAAS blockade with stand-alone ACE inhibition.43–45 Clinical trials have demonstrated that mineralocorticoid receptor antagonists (MRAs) can improve prognosis in addition to standard therapy with ACE inhibitors and beta-blockers.46,47 MRAs likely promote antifibrotic actions in a broad range of organs such as the heart, kidney, vasculature and lungs, all of which are affected in HF. Despite their class I indication, however, they remain markedly underutilised in daily HF practice, probably due to their real and perceived potential off-target effects on renal function and serum potassium levels.4,48,49 Following encouraging preclinical studies, non-steroidal MRAs are currently being investigated in clinical trials such as the MinerAlocorticoid Receptor antagonist Tolerability Study – Heart Failure (ARTS-HF).14,50–53 These novel compounds appear to induce less hyperkalaemia and less worsening of renal function in HF.
Dual-acting Neprilysin/RAAS Blockers: from Omapatrilat to Sacubitril/Valsartan
The natriuretic peptide (NP) system promotes natriuresis and diuresis and lowers blood pressure. In HF patients, the NP system also counteracts the RAAS and SNS, thereby attenuating the hypertrophy and fibrosis of CV and renal tissues as well as inflammation and neo-angiogenesis.54–56 Hydrolysis by the metallopeptidase neprilysin constitutes the primary breakdown mechanism of NPs; therefore pharmacological targeting of neprilysin has been proposed as a strategy to restore or augment the beneficial actions of NP.57 Singleacting neprilysin inhibitors produce essentially neutral effects in humans, perhaps due to the fact that neprilysin broadly interacts with other vasoactive peptides such as adrenomedullin, bradykinin, endothelin-1, substance P, encephalin and others.58–60 Apart from the membrane-bound fraction of neprilysin, a soluble form exists that is measurable and retains activity in the plasma of patients with HF.61 Better understanding of the molecular mechanisms underlying HF has led to the recognition that in order to exploit the benefits of neprilysin inhibition, RAAS needs to be inhibited concomitantly.62
The vasopeptidase inhibitors were the first class of drugs to inhibit both ACE and neural endopeptidase.63,64 Omapatrilat underwent extensive clinical testing in the treatment of hypertension and HF.65,66 Omapatrilat showed superior antihypertensive effects to stand-alone RAAS blockade in the large Omapatrilat Cardiovascular Treatment versus Enalapril (OCTAVE) trial (n=25,302).67 In the phase-II Inhibition of Metallo Protease by BMS-186716 in a Randomized Exercise and Symptoms Study in Subjects With Heart Failure (IMPRESS) trial, omapatrilat reduced the composite endpoint of all-cause mortality or HF hospitalisation compared to lisinopril.68 In the subsequent phase-III Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE), however, the primary endpoint was not significantly reduced and the trial failed to meet the pre-specified superiority criterion.69 Important off-target effects, most notably a substantially higher rate of angioedema ascribed to bradykinin accumulation, halted further development of omapatrilat and other vasopeptidase inhibitors.70
A logical extension of research efforts into combined neprilysin and RAAS blockade are the angiotensin receptor neprilysin inhibitors (ARNIs).62,71,72 Utilising an ARB rather than ACE inhibitor as the RAAS blocker, ARNIs circumvent the issue of bradykinin accumulation.70 In an experimental angioedema model, vasopeptidase inhibition – but not ARB or neural endopeptidase inhibition or their combination (replicating ARNIs) – induced bradykinin-mediated tracheal plasma extravasation.73 Sacubitril/valsartan, the first-in-class ARNI, has undergone broad clinical testing in HF and hypertension.74,75 The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial evaluated sacubitril/valsartan as an alternative to enalapril in patients with HFrEF (i.e. current best therapy based on the Studies Of Left Ventricular Dysfunction (SOLVD) study).76,77 The trial was terminated prematurely due to overwhelming benefit: compared to enalapril, sacubitril/valsartan reduced the risk of the primary composite endpoint of CV mortality or hospitalisation for HF by 20 %. Sacubitril/valsartan was also superior in reducing a number of other pre-specified endpoints, such as time to clinical deterioration and 30-day readmission rates, and was more efficacious regardless of age, LVEF or the presence of AF.78–82 Experimental work suggests that sacubitril/valsartan better protects against angiotensin-II-stimulated myocardial cellular injury, hypertrophy and fibrosis than single-acting RAAS blockade.83,84 Such dual-acting neurohormonal inhibition was also recently reported to offer better renal protection compared to single RAAS blockade.85–87 Based on encouraging results from the phase II Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT-HF) study, sacubitril/valsartan is currently being tested in Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF), a large clinical outcome trial scheduled to enrol 4,300 patients with HFpEF (www.clinicaltrials.gov, NCT01920711).75
Other Neurohormonal Systems with Possible Relevance to HF
Endothelin-1, the major isoform of the endothelin peptide family in the CV system, is an extremely potent vasoconstrictor with additional pro-hypertrophic, pro-fibrotic and mitogenic effects on myocardium and vasculature.88 Endothelin activation in HF disturbs salt and water homeostasis, stimulates the RAAS and SNS, mediates vasoconstriction, and directly contributes to progressive CV and renal dysfunction and remodelling in HF.89,90 Endothelin-1 plasma levels are strongly correlated with mortality and morbidity.91
Fuelled by encouraging experimental and early clinical evidence, several RCTs have explored the putative utility of blocking the endothelin system in acute and chronic HF settings.90,92–96 With the exception of some forms of pulmonary arterial hypertension, the vast majority of large RCTs of endothelin antagonism have failed to show reduced clinical event rates (see Table 1). Unfortunately, some trials (with neutral or negative outcomes presented at scientific meetings) have not been published, or only in abstract form.97–100 In HF, the only current application of endothelin antagonists seems to be to lower pulmonary vascular resistance in high-risk patients on the heart transplant list, although even this indication has been subject of debate.101–103
Among numerous other neurohormones with putative implications in HF pathophysiology are adrenomedullin, bradykinin, serotonin, and urotensin-II.104–107 Their role in HF remains incompletely understood, and no specific pharmacological modulator has advanced into clinical testing. Since several of these neurohormones are substrates of neprilysin, their metabolism could conceivably be altered by neprilysin inhibition.58–60
Remaining Challenges for Neurohormonal Blockade in HF
Concomitant blockade of multiple neurohormonal systems, built on a strong scientific foundation, is the current gold standard of pharmacotherapy in HFrEF. Current treatment recommendations are based on trials that showed clinical benefits for target doses of RAAS and SNS blockers.4 Guideline-adherent treatment is frequently not achieved in practice, however.108 There are various reasons for this, such as the lenient attitude of some caregivers (sometimes termed “therapeutic inertia”) towards patients who appear euvolemic and asymptomatic, and the real or perceived side effects of medical therapy such as hypotension, bradycardia, hyperkalaemia and worsening renal function.109
There is a considerable knowledge gap regarding neurohormonal blockade in various HF entities: renal dysfunction affects at least one in five HF patients and is a major adverse prognostic factor.110 Traditionally these patients have been excluded from RCTs, although there is accumulating evidence for the particular value of neurohormonal blockade in these patients, as discussed above. HF commonly coexists with AF and represents a clinical dilemma.111,112 In patients with HFrEF and AF, the mortality and morbidity benefits of beta-blockers for neurohormonal blockade appear to be absent,15,21 and the data for RAAS antagonists and MRAs are limited.111 Patterns of autonomic activation have not yet been sufficiently studied in patients with concomitant HF and AF, limiting our understanding of the impact of pharmacotherapy.
Some authors have argued that the therapeutic blockade of neurohormonal systems may have been exhausted, and that a ceiling may have been reached. In particular, a discrepancy between promising early-phase and frequently disappointing clinical endpoint trial results of neurohormonal blockade has been noted.113 Recent examples of neurohormonal blockers with promising scientific underpinnings that failed to lower event rates in clinical early-phase or outcome trials include endothelin receptor blockers, adenosine receptor antagonists, tumour necrosis factor antagonists and phosphodiesterase inhibitors.98,114–116 Of note, very recent insights from the PARADIGM-HF study using valsartan/sacubitril support the notion that combination therapy with neurohormonal modulators may be superior to singleacting therapy, even at subtarget doses.117 Such a strategy may better exploit the benefits of abrogating multiple specific maladaptive signalling pathways while circumventing the adverse effects of neurohormonal blocker monotherapy. For instance, renal failure frequently occurs in HF patients, and experimental as well as clinical studies have demonstrated that dual-acting RAAS blockade and neprilysin inhibition offers superior nephroprotection to single-acting therapy.85,86
Finally, no single effective therapy has been identified for patients with HFpEF,118 although this category includes a very heterogeneous population defined by an arbitrary cut-off in LVEF. The limited benefit of neurohormonal blockers in HFpEF may also be explained by older age, more advanced comorbidities and a higher likelihood of death from non-CV causes.119–121 Rates of AF are also higher in patients with HFpEF, leading to additional neurohormonal activation.111,112 In addition, a substantial proportion of patients with HFpEF show evidence of impaired or resolving systolic function.122 The recently-introduced category of HF with midrange ejection fraction has little evidence-base as yet, but will likely increase clinical awareness of these patients.
Conclusion
Sustained activation of neurohormonal systems is a hallmark feature of HF. The clinical use of neurohormonal blockers has revolutionised the care of patients over the past four decades. Drug therapy that is active against imbalance in both the autonomic and renin– angiotensin–aldosterone systems consistently reduces morbidity and mortality in chronic HF with reduced LVEF and sinus rhythm. HF is an extraordinarily complex and multi-faceted chronic syndrome, and current knowledge of the interface between the epidemiological, clinical, pathophysiological and molecular features remains limited. Initiation and up-titration of effective neurohormonal therapies remains challenging in patient subcohorts. In addition, optimal medical therapy is frequently not achieved or even attempted despite HF having a similar overall prognosis to cancer. The recent introduction of the novel ARNI drug class attests to superior efficacy of multiple-acting neurohormonal blockade in chronic HF. HFpEF and HF with coexisting AF represent major remaining clinical challenges that appear to be less susceptible to conventional pharmacotherapy. Novel neurohormonal blockers and the refined use of existing therapeutic agents, as well as up-titration to recommended target doses, are needed to reduce adverse clinical events and to improve outcomes in HF.