Heart failure is a clinical syndrome induced by cardiac abnormalities resulting in reduced cardiac output and/or elevated intra-cardiac end-diastolic pressures and causing symptoms that are often accompanied by typical physical signs.1 Demographic changes, improved treatment of several acute cardiac disorders, such myocardial infarction, arrhythmia and congenital heart disease, and increased long-term survival of patients with reduced left ventricular systolic function have led to a dramatic increase in the number of patients living with heart failure.
Acute heart failure (AHF) is defined as new-onset or worsening of symptoms and signs of heart failure.1 AHF is the most frequent cause of unplanned hospital admission in patients aged 65 years or older and is characterised by significant in-hospital mortality and frequent readmissions.2,3 Outcomes of AHF remain globally poor.4,5 Independent of ejection fraction, the average survival after hospitalisation for AHF is 2 years, with the most vulnerable phase being in the months directly after discharge from hospital. Despite significant achievements in the treatment of chronic heart failure, all trials targeting AHF with short-term in-hospital therapies have had disappointing or at most neutral endpoints. Thus, the optimal strategy for improving long-term outcomes in patients admitted with AHF should be revisited. Optimisation, personalisation and continuation of (A)HF treatment after hospital discharge seem crucial to achieving the best outcomes. This article reviews the principles of optimisation and personalisation of AHF treatment during the hospital stay and early outpatient phase.
Triage and the Initial 7-P Evaluation
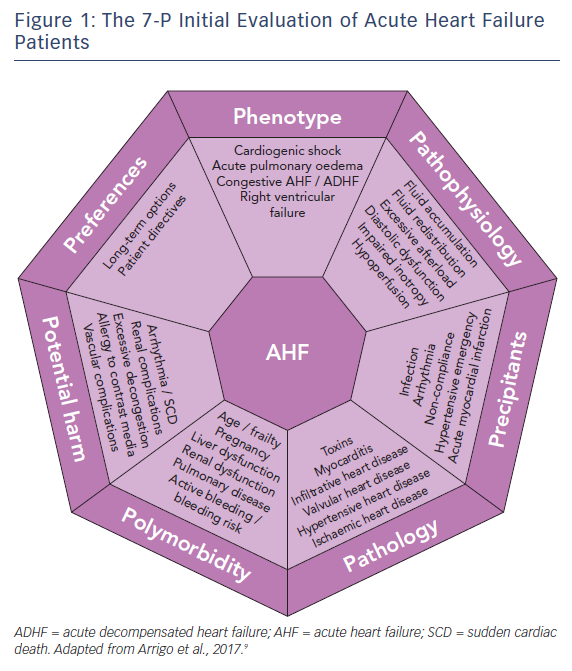
Patients presenting with (suspected) AHF should undergo rapid triage to exclude cardiogenic shock, respiratory failure, myocardial infarction and/or arrhythmia and receive the appropriate level of monitoring and specific treatments (e.g. pharmacological/mechanical haemodynamic support, mechanical ventilation and percutaneous revascularisation).1,6,7 Moreover, since AHF is a life-threatening condition, initial treatment should be started as soon as possible, ideally within 30–60 min after hospital admission, as this is associated with better outcomes.6–8 The initial treatment should then be tailored and optimised according to a 7-P evaluation: phenotype, pathophysiology, precipitants, pathology, polymorbidity, potential harm and preferences,9 see Figure 1.
Phenotype
The initial evaluation should include the assessment of the clinical phenotype based on symptoms or signs of peripheral hypoperfusion (forward failure) and/or systemic congestion (backward failure). However, given the limited sensitivity and specificity of clinical assessment, additional confirmatory tests may be required, such as biomarkers, echocardiography, lung ultrasound or chest X-ray, to exclude differential diagnoses.
The vast majority of AHF patients are well perfused but congested (warm-wet), while only a minority are hypoperfused (either cold-wet or cold-dry). Hypoperfusion defines cardiogenic shock, the most severe clinical presentation of AHF, which accounts for only about 10 % of AHF cases. However, the treatment of these patients is often more difficult and is associated with 5- to 10-fold higher in-hospital mortality compared to normally perfused cases.4,10 Systemic congestion, in contrast, is widespread and results from the combination of fluid accumulation and redistribution due to a change in vascular compliance, with variable proportions according to the clinical scenario. Fluid accumulation is found predominantly in cases of acute decompensation in chronic heart failure with reduced systolic function, while fluid redistribution mostly occurs in new-onset AHF patients with preserved systolic function and/or systemic inflammation.
Pathophysiology
The second step in the evaluation of AHF patients should focus on understanding the leading pathophysiology at play. AHF can be a consequence of arrhythmia (with and without atrio- or inter-ventricular asynchrony), anatomical defects, incompetent valves, impaired myocardial contractility, pathological myocardial relaxation, hampered ventricular filling and/or excessive cardiac afterload.
As already mentioned, systemic congestion is the central feature of AHF and results from the combination of fluid accumulation and redistribution induced by neurohumoral activation in the presence of cardiac dysfunction.11 Hypoperfusion manifests only in the most severe forms of AHF (cardiogenic shock) in the presence of severely impaired cardiac output.12 In patients without a previous history of symptomatic heart failure (de novo AHF), AHF mostly occurs secondary to a sudden deterioration in cardiac function – due to myocardial infarction, severe myocarditis or acute valve regurgitation, for example – causing fluid redistribution and, in severe forms, peripheral hypoperfusion.3 These patients have no or only minor increases in body weight before hospital admission. Fluid redistribution and loss due to sweating, perspiratio insensibilis or diuretic therapy can cause intravascular hypovolaemia and insufficient preload. Consequent sympathetic activation induces transient vasoconstriction leading to rapid volume displacement from the peripheral and splanchnic venous systems to the pulmonary circulation.13,14 A mismatch in the ventricular–arterial coupling relationship, with increased afterload and decreased venous capacitance (increased preload), is the primary alteration in hypertensive AHF.15,16
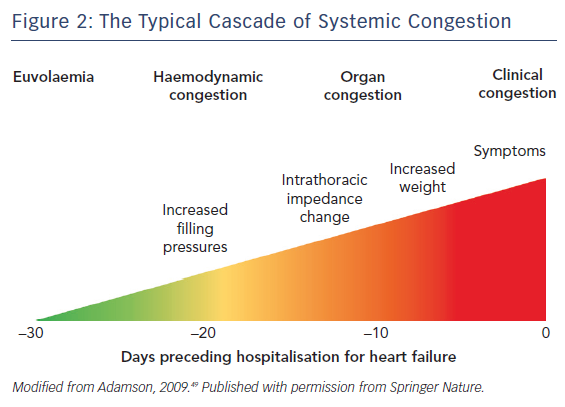
More frequently, AHF consists of acute decompensation of chronic heart failure (ADHF) and is caused by progressive fluid accumulation. Indeed, persistent neurohumoral activation impairs renal sodium excretion, resulting in sodium and then fluid accumulation.17 The classical congestive cascade includes subclinical stages characterised by increased cardiac filling and venous pressures (haemodynamic congestion), followed by redistribution of fluids into the lungs and visceral organs (organ congestion) and finally to overt symptoms and signs of volume overload (clinical congestion), see Figure 2.11 Although clinical and organ congestion usually follows haemodynamic congestion, the correlation between hydrostatic pressure and oedema formation is weak. Indeed, chronic sodium accumulation in heart failure impairs the function of the interstitial glycosaminoglycan network, reducing its capacity to buffer additional sodium and maintain low interstitial compliance.18 Consequently, interstitial oedema formation may occur even in the presence of mildly elevated hydrostatic pressures and vascular capacitance may change, causing increased cardiac filling pressures without increments in vascular or total body fluid.
Precipitants
During the third step, triggers of AHF should be identified. AHF may be precipitated by several factors that may coexist, such as myocardial ischaemia, arrhythmias, infections, uncontrolled hypertension and non-compliance with medical prescriptions.2,19,20 In patients presenting with cardiogenic shock, myocardial infarction is by far the most common precipitant.10 The aims when identifying precipitants are to detect treatable causes (see below) and provide prognostic information. AHF precipitated by an acute coronary syndrome or infection is associated with poorer outcomes; whereas outcomes tend to be better in AHF precipitated by atrial fibrillation or uncontrolled hypertension.2,20,21
Pathology
The initial treatment of AHF should be started as soon as possible according to the clinical presentation. However, understanding of the underlying cardiac pathology is essential for providing optimal specific therapy and estimating prognosis. For example, giant cell myocarditis requires aggressive immunosuppressive therapy, while severe mitral regurgitation caused by papillary muscle rupture requires cardiac surgery.22 Moreover, end-stage ischaemic heart disease without reversible ischaemia and viability may display significantly lower recovery potential than peripartum cardiomyopathy.23 Infiltrative heart disease may involve other organ systems. Immediate echocardiography is recommended in patients presenting with cardiogenic shock or de novo AHF.1,6
Polymorbidity
AHF is a syndrome causing organ dysfunction, mainly of the lungs and abdominal organs.24–26 Historically, renal and hepatic dysfunctions in heart failure have been considered the consequence of visceral hypoperfusion, but more recent data have shown that venous congestion is the strongest haemodynamic determinant of renal and hepatic dysfunction in AHF.27,28 Assessment of organ dysfunction – in particular severe renal and kidney failure – as well as other conditions causing relative contraindications to diagnostics or treatment – such as allergy, pregnancy or active bleeding – are crucial in deciding on optimal diagnostic modalities and treatments to be delivered. Rapid assessment of frailty is recommended in geriatric patients, since it affects overall outcome.29,30 Metabolic disturbances, such as diabetes or thyroid disease, anaemia and iron deficiency should be assessed and optimised.
Potential Harm
It is crucial to consider the risk of iatrogenic harm associated with diagnostics and treatment in every medical decision made. This is even more important in the treatment of AHF patients – a population of older, critically-ill, polymorbid subjects.31 For example, indiscriminate use of diagnostics (e.g. coronary angiography) and monitoring (e.g. pulmonary artery catheter) may expose patients to severe vascular or radiological complications; excessive use of inotropic agents in the absence of evidence of peripheral hypoperfusion is associated with arrhythmia and excess mortality.32,33
Patient Preferences
The seventh part of the initial evaluation of AHF patients should focus on patient preferences and ethical issues. Discussion with the patient (if feasible) or with relatives about resuscitation directives and treatment options may be time-consuming but is crucial to avoid overtreatment. Importantly, long-term options, such as mechanical assist devices or transplantation, and the wishes of the patient need to be evaluated early rather than late, particularly in AHF patients with the potential for rapid deterioration. In the absence of long-term therapeutic options, palliation and supportive care should be offered to patients. Relatives should be advised of these options if patients are not in a position to consent.34
Treatment at Hospital Admission
Correctly deciding which phenotype/pathophysiology predominates is critical in determining which treatment strategy should be used.35 At hospital admission, AHF patients displaying evidence of congestion should receive decongestive treatment such as vasodilators and/or diuretics.8,36 While diuretics are mainly used in the presence of fluid overload, vasodilators are administered to reduce filling pressures in the presence of fluid redistribution and preserved systolic blood pressure (>110 mmHg; more cautiously between 90 and 110 mmHg). Decongestive therapy should be started as soon as possible and titrated according to clinical response.8 Notably, decongestive therapy should be continued beyond the improvement of symptoms and clinical evidence of organ congestion and maintained until euvolaemia is achieved, see Figure 2.
The use of inotropes should be restricted to patients in cardiogenic shock due to impaired myocardial contractility, since their inappropriate use is associated with increased morbidity and mortality.37 In cases of persistent haemodynamic instability despite escalating doses of inotropes, mechanical circulatory support such as veno–arterial extracorporeal life support and percutaneous left-ventricular assist devices should be considered before irreversible organ failure has established.7 In severe pulmonary oedema causing hypoxia, high-flow oxygen therapy, non-invasive or invasive mechanical ventilation are required to ensure oxygenation.
In addition to decongestive therapy, initial management should include specific treatments directed towards decompensation triggers and the underlying cardiac disorders. In particular, early coronary angiography with revascularisation is recommended in AHF precipitated by acute coronary syndrome. Antiarrhythmic treatment and/or electrical cardioversion are recommended in AHF precipitated by arrhythmia. Rapid initiation of antimicrobial therapy is recommended for AHF precipitated by infection/sepsis. Sometimes, percutaneous or surgical treatment of structural heart disease is required to achieve durable stabilisation. Finally, patients should be maintained on oral disease-modifying heart failure treatment whenever possible.1
After delivery of the initial treatment, continuous reassessment of clinical response and patient allocation in terms of level of care should be ensured. The level of care (discharge home, observation, ward, telemetry or intensive/intermediate care unit) should integrate symptom severity, precipitating factors, haemodynamic and respiratory status, the degree of congestion and biomarkers (i.e. natriuretic peptides, troponin, renal function and serum lactate) and the patient’s general condition. Most patients require hospital admission, about half of them to intensive or intermediate care units. Low-risk patients with good response to initial therapy may be considered for early discharge.
Treatment Before Discharge and the First Outpatient Visit
The optimal time-point for discharging hospitalised AHF patients may be difficult to determine due to the need to balance patient preferences, healthcare resources and the risk of adverse outcomes. Indeed, the risk of death is high during hospitalisation for AHF but is even higher during the immediate post-discharge period, which usually lasts 2–3 months and is known as the vulnerable phase.38 Therefore, optimal transitions of care after hospital discharge may be even more important than the delivery of appropriate treatments during hospitalisation in reducing adverse outcomes in AHF patients.
Since the causes of the vulnerable phase remain controversial, identification of patients at particularly high risk of adverse outcomes after hospital discharge is particularly challenging. A combination of pathophysiological disorders and lack of follow up seems to contribute to the high mortality and readmission rates observed. Several risk scores using multiple clinical variables have been developed, but most of them are complex and lack accuracy. Cardiovascular biomarkers added to clinical parameters may reveal active sub-clinical processes, providing valuable insights into the pathophysiology of the vulnerable phase and increasing prognostic accuracy. In the future, a comprehensive multi-marker strategy reflecting different activated pathways in heart failure (myocardial stress, myocyte injury, neurohumoral activation, inflammation, oxidative stress, matrix remodelling and systemic congestion) may increase the precision of biomarker-guided prognostication.39 Even more importantly, the prognostic information derived from a single or multi-marker strategy may be translated into therapeutic decisions and personalisation of follow up, improving patient outcomes.
Persistent subclinical congestion may contribute to the high rates of death and readmission observed after hospital discharge.40 Indeed, despite a global improvement in symptoms during hospital stay, a relevant proportion of patients still display markedly elevated natriuretic peptides at discharge. This discordance between few symptoms and high natriuretic peptide concentrations suggests persistent haemodynamic congestion. Some studies have reported an association between pre-discharge levels of natriuretic peptides and subsequent risk of death or readmission.41 Based on these data, titration of decongestive therapy based only on symptoms and signs may be insufficient and should include additional parameters such as biomarkers and/or echocardiography.42,43
Underutilisation of disease-modifying heart failure therapies such as beta-blockers, renin–angiotensin system (RAS) inhibitors and mineralocorticoid receptor antagonists is prevalent and may further promote adverse events after hospital discharge.5,44 Beta-blocker discontinuation during hospitalisation is associated with detrimental effects on short-term mortality and readmission.45 Very recently, a large propensity score-matched cohort study showed an association between beta-blocker or RAS inhibitor treatment at hospital discharge and a 40–50 % relative risk reduction in 90-day mortality.44 It showed an additional 25–50 % relative risk reduction with combined beta-blocker and RAS inhibitor therapy at hospital discharge compared to either treatment alone.44 The early benefits were present in both reduced and preserved ejection fraction and persisted at 1-year follow up. In the same study, no significant benefit was found with early mineralocorticoid receptor antagonist administration. In beta-blocker-intolerant patients, early administration of ivabradine might be considered to reduce readmissions during the vulnerable phase.46 Current European Society of Cardiology guidelines recommend initiation or continuation of disease-modifying heart failure therapies during hospitalisation in all AHF patients with reduced ejection fraction unless contraindicated.1 Similarly, evaluation of the patient to determine whether a cardiac device (implantable cardiac defibrillator and/or cardiac resynchronisation therapy) is indicated and, if so, planning for its implantation should not be overlooked.
Furthermore, hospital discharge should occur only after precipitating factors of AHF have been adequately treated and resolved. This point includes revascularisation for myocardial infarction, antiarrhythmic therapy for arrhythmias, antimicrobial treatment for infection, antihypertensive therapy for hypertension and patient education for non-compliance with recommendations. Patient education, home-based measurement of body weight and blood pressure, and early contact with healthcare providers have all been proposed to reduce readmission rates; however, these interventions have produced inconsistent results. Early detection of increasing congestion with intrathoracic impedance monitoring and implantable haemodynamic monitoring, e.g. with the CardioMEMS™ HF System (St Jude Medical), have shown promising results in trials but concerns about their cost-effectiveness has prevented their widespread introduction in clinical practice.47,48
Finally, hospital discharge should be planned to allow inclusion of all patients into a comprehensive, post-discharge care programme. If this is not feasible because of limited resources, entry to such a programme should be restricted to those with high-risk features such as markedly elevated natriuretic peptides, abnormal systolic blood pressure, persistent hyponatraemia and recurrent readmissions.38 Outpatient visits to a general practitioner and heart failure clinic should be scheduled before hospital discharge to ensure appropriate follow up. In our centres, we usually plan a visit to the general practitioner within 1 week and a visit to the heart failure clinic within 2–3 weeks after discharge.
During early outpatient visits, assessment and optimisation of volume status (including the comparison of natriuretic peptide concentration with pre-discharge values), up-titration of disease-modifying heart failure treatment and evaluation of cardiac device indication should be performed. After bridging the vulnerable phase, optimisation of oral disease-modifying treatments and regular follow-up visits should be continued on an individual basis.
Conclusion
The initial therapy of AHF should be personalised based on clinical phenotype, the pathophysiology at play, precipitants identified and underlying cardiac pathology. Particular attention should be given to polymorbidity, including organ dysfunction, and the avoidance of potential iatrogenic harm. Patient preferences and ethical issues should be integrated into the treatment plan at an early phase. Before hospital discharge, persistent subclinical congestion and underutilisation of disease-modifying heart failure therapies should be addressed and appropriate follow up ensured.