Postpartum cardiomyopathy (PPCM) is a diagnosis of exclusion, where patients present with heart failure secondary to left ventricular (LV) systolic dysfunction towards the end of pregnancy or in the months following delivery, with no other cause of heart failure identified.1 PPCM is relatively uncommon, affecting between one in 5,000 and one in 10,000 births;2 it is thought to be more prevalent in women aged over 30 years, of black ethnicity, with a history of pre-eclampsia or pregnancy-induced hypertension and those who have had multiple gestations.3
Symptoms can be indistinguishable from those of normal pregnancy, with women presenting with dyspnoea, orthopnoea and reduced exercise capacity. Physical examination, chest X-ray and echocardiography are generally consistent with a diagnosis of heart failure, and levels of brain natriuretic peptide (BNP) and troponin are usually elevated. Clinical progression varies considerably, with some patients advancing to end-stage heart failure within a few days of presentation. Similarly, recovery of left ventricular function is variable and can occur spontaneously.1
Management of acute and stable heart failure in PPCM should focus on control of volume status, dampening neurohormonal responses and reducing the risk of associated thromboembolic and arrhythmic complications.4
While treatment pathways for heart failure are well established, the implications for infants whose mothers choose to breastfeed while on heart failure pharmacotherapy need to be considered. Appropriate treatment for the mother must be a primary concern, with breastfeeding compatibility a secondary consideration.
A recent practical guide on peripartum cardiomyopathy1 considers the use of bromocriptine (in addition to standard therapy for heart failure) depending on the extent of presenting LV function. Bromocriptine will effectively stop lactation and the implications of this should be discussed with the mother should this treatment option be required. Evidence for the effectiveness of bromocriptine in PPCM comes from relatively small studies with many confounding variables. The on- going EURObservational Programme on PPCM5 is anticipated to provide longer term outcome data and may give clearer insights on the place of bromocriptine in the management of PPCM.
The benefits of breastfeeding to both mother and infant are recognised,6 so it is important to protect the breastfeeding relationship wherever possible; advising a mother not to breastfeed because of medicine exposure is not a ‘zero risk’ option to either mother or infant. As well as breast milk being a tailored nutrition source,7,8 other immediate benefits to the infant include immunoprotection7,9 and pain relief for procedures such as heel prick.10 Longer-term benefits to the infant include reduced risk of sudden infant death,11 a lower risk of becoming obese,12,13 and improved cognitive development.14,15 Benefits to the mother include reduced risks of breast cancer,16,17 ovarian cancer,18 type II diabetes19 and hypertension.20
Documented evidence for the use of medicines during breastfeeding is largely very poor, if available at all, often leading to an incorrect decision that the mother should not breastfeed. However, any evidence available can be used, along with a consideration of drug properties and pharmacokinetics, to carry out an informed risk assessment. In most cases, medicine use can continue during breastfeeding, and most risks can be managed.
On the very rare occasions where breastfeeding cannot be recommended becaues of medicine exposure, an abstinence period will be required to ensure that the medicine has been excreted out of the breast milk compartment. The mother may also be too acutely unwell to physically breastfeed. In either scenario, the mother will need to express her milk to maintain supply so she can start breastfeeding at a later stage if she wishes. If an abstinence period has been recommended due to medicine exposure, expressed milk will need to be discarded. Otherwise, expressed milk can continue to be given to the infant.
The licensed use of medicines is set out in their summary of product characteristics. This provides information from the manufacturer on a medicine and conditions attached to its use. A section is included on use in breastfeeding but this is often overcautious and recommendations are based on the lack of evidence, rather than a more considered risk assessment. To date, there is no requirement for manufacturers to conduct studies in breastfeeding since, understandably, it would be unethical to do so.
The advice presented in this article takes into account known pharmacokinetic principles, clinical experience to date and, where available, published evidence.
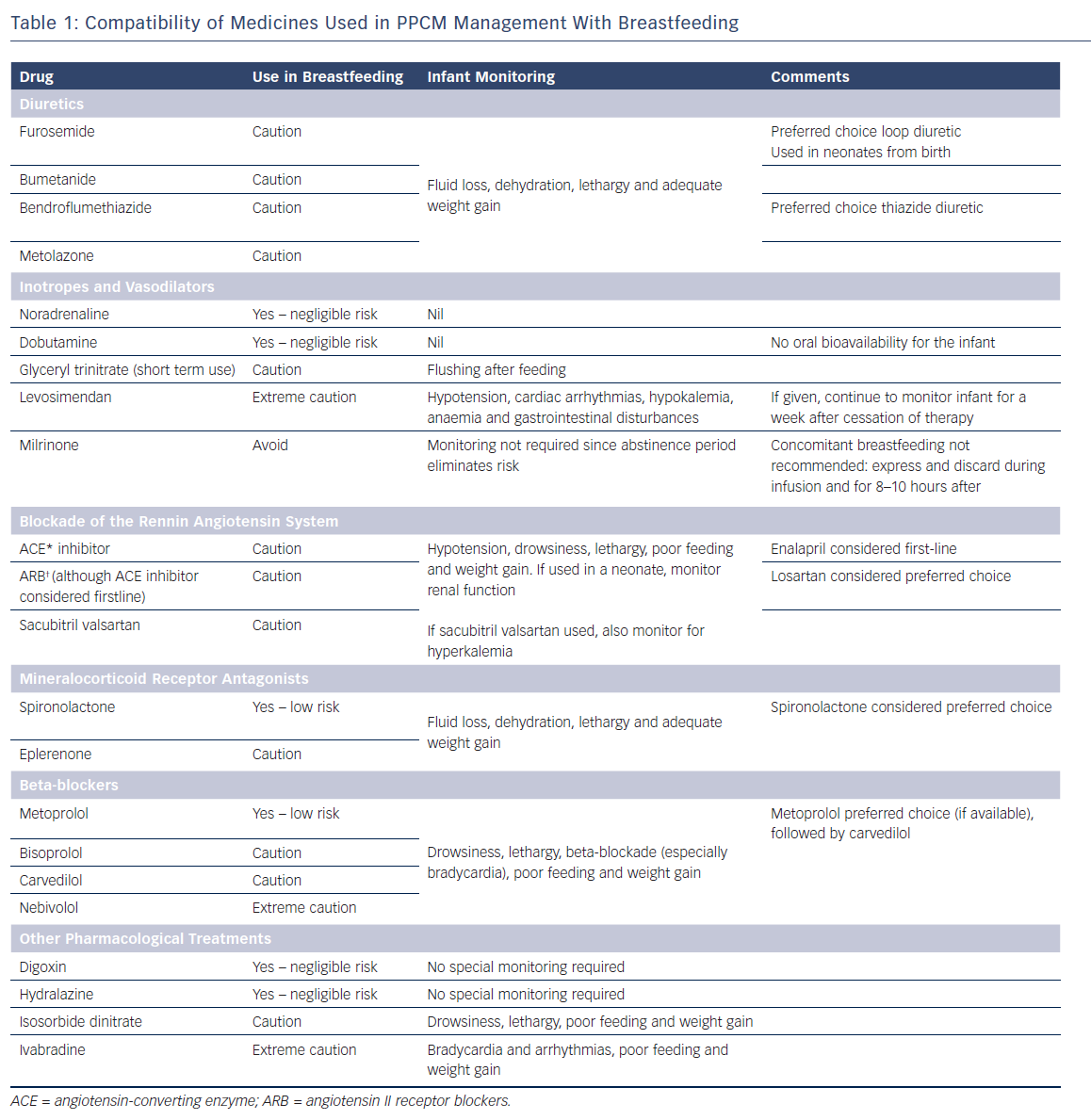
Timing of feeds against a given dose is not generally advocated, since there is no evidence to suggest what additional benefit this practice confers, and it can be stressful for a mother to adhere to these conditions, especially when she is taking multiple medicines. Therefore the risk assessments presented within this article represent the overall on-going exposure of the breastfed infant. Advice is offered in supporting breastfeeding wherever possible, but it should be noted this may be contrary to advice given by the manufacturers and would render the product being used outside its product license. Unless otherwise stated, throughout this article, there are no published data for the use of medicines used in PPCM management during breastfeeding. Table 1 provides a useful and concise summary.
Principles of Determining Drug Safety in Breastfeeding
Nearly all medicines will pass into breast milk to some extent, although transfer is usually low. Many factors affect milk drug concentration: these can be drug related (dose, bioavailability, lipid solubility, protein binding, molecular weight, pKa, half-life, active metabolites and mechanism of elimination); and maternal related (maternal dose, pharmacogenomics and renal and hepatic function). In addition, the adverse effect profile of the medicine needs to be considered since these can potentially occur in the exposed infant.
Common principles are used to help in risk assessment for compatibility of a medicine during breastfeeding include (note this list is not exhaustive):
- Protein binding If a drug is highly protein bound, less fraction of the drug is freely available to pass across into breast milk.
- Half-life Drugs with longer half-lives have a greater potential to accumulate in the infant, which increases the risk of the infant experiencing side effects. Half-lives of active metabolites also need to be considered.
- Oral bioavailability If a drug has minimal or low oral bioavailability (for example drugs administered via parenteral routes to achieve good therapeutic concentrations), when the infant ingests the drug orally via breast milk, it will not lead to significant systemic concentrations in the infant.
- Lipid solubility Drugs that have high lipid solubility are more likely to pass into breast milk because they are able to pass through the lipid membrane of the alveolar epithelium.
- Molecular weight Small molecules are more likely to be able to pass into breast milk via passive diffusion. Larger molecules require other transporting mechanisms or will simply be too big to pass through. It is generally accepted that molecules with a molecular weight above 1,000 will be able to pass through only in small quantities if at all.7 Very large molecules, such as proteins, are generally considered too large to pass through into breast milk, although evidence to the contrary is beginning to emerge.21,22
- Infant age Newborn infants, especially premature infants, have underdeveloped hepatic and renal clearance capacities, which can lead to accumulation. An older infant will have better developed clearance pathways. Additionally, once an infant is being weaned, overall milk consumption (and hence drug exposure) will lessen.
- Paediatric use If a drug is used therapeutically in infants, there is experience of its use in the paediatric population, which provides reassurance of the drug’s acceptability in breastfeeding since the level of exposure from breast milk is generally much smaller than that used to achieve a therapeutic effect.
The clinical status of the infant needs to be taken into consideration. If an infant is unwell, effects from drug exposure in breast milk need to be considered to avoid a potential deterioration in their condition. A premature infant is far more likely to be vulnerable to effects from medicines because of the increased risk of drug accumulation from underdeveloped clearance capacities. If either of these situations apply, it would be advisable to seek specialist advice since an individual risk assessment on the infant should be carried out.
It is usually advised that medicines used during breastfeeding should be at the lowest effective dose for the mother and for the shortest duration possible to minimise exposure of medicines to the infant through breast milk. However, in the treatment of PPCM, the mother should receive the treatment she requires, often with dosing being maximised for optimal effect, with safety in breastfeeding being the secondary consideration.
Pharmacotherapy in Acute Heart Failure Secondary to Postpartum Cardiomyopathy
Acute heart failure requires urgent admission to hospital for assessment and offloading of volume, which usually requires both intravenous diuretics and glyceryl trinitrate for vasodilation, both of which are titrated to response and systolic blood pressure. Patients with a low output state or with persistent congestion may need ionotropic agents, namely noradrenaline, dobutamine, milrinone or levosimendan, until organ perfusion is restored and/or congestion reduced.
Loop diuretics (furosemide and bumetanide) have favourable pharmacokinetic properties so levels in breast milk are anticipated to be low. They have high protein binding (up to 99 %) and are short acting (half-life of approximately 2 hours).
Furosemide is considered to be the preferred choice loop diuretic during breastfeeding since it has lower oral bioavailability (60–70 %) than bumetanide (80–95 %); oral bioavailability of furosemide is even lower in neonates.23 Furosemide is also used in full-term neonates from birth.24
Patients with significant fluid overload or failure to achieve adequate diuresis with a single loop diuretic may require the addition of a thiazide diuretic such as bendroflumethiazide or metolazone. As with loop diuretics, the pharmacokinetic profile of thiazides predicts that levels expressed in breast milk would be too low to have an effect in the infant.25 Bendroflumethiazide is the preferred choice, as there is more experience of its use and because of its pharmacokinetic profile (>90 % protein binding and short acting, with a half-life of 3–4 hours).23,26
Although the amounts of loop and thiazide diuretic predicted to cross into breast milk are not thought to be harmful, there is concern that intense diuresis may suppress lactation.27,28 If high doses or combinations are used, the mother’s milk supply should be monitored by ensuring the infant is gaining weight adequately and monitoring the infant for fluid loss, dehydration, and lethargy.25
Vasodilation with glyceryl trinitrate can help to reduce pre and post load in the myocardium and, as such, offers value in resolving symptoms in acute decompensated heart failure. As it has a very short half-life of minutes,23 the drug’s passage into breast milk would not be expected at clinically relevant concentrations. Although short-term use is considered compatible with breastfeeding, the infant should be monitored for signs of nitrate absorption such as flushing after feeding.25
Where inotropic support is necessary to maintain adequate cardiac output, noradrenaline, dobutamine, milrinone or levosimendan may be used. Noradrenaline is extremely short acting29 with a half-life of 1–2 minutes so breast milk levels would be expected to be very low. In addition, due to negligible oral bioavailability, the infant is not predicted to absorb any from their gastrointestinal tract. There is some concern from animal data that very high doses may interfere with the lactation process itself,30,31 although there is no clinical evidence to support this. Therefore, noradrenaline can be used during breastfeeding without any special precautions. Similarly, dobutamine is considered compatible due to favourable pharmacokinetic properties, including an extremely short half-life of around 2 minutes and negligible oral bioavailability.22
Milrinone with a low molecular weight (211) and protein binding (70 %) would suggest passage into breast milk.23 Despite it being given intravenously, it is almost completely absorbed after oral administration23 suggesting the infant will absorb what they are exposed to. Milrinone has a relatively short half-life of 2.3 hours, and is generally given in the acute phase to those unresponsive to other treatments. Because of its pharmacokinetics and adverse effect profile, it is advisable to temporarily disrupt breastfeeding during a milrinone infusion and for 8–10 hours after the infusion has been completed.25 The mother should be encouraged to express and discard breast milk during this time.
Levosimendan has good oral bioavailability (85 %)32 and is extensively metabolised, with a short half-life of about one hour although its active metabolites (OR–1855 and OR–1896) have longer half-lives (75–80 hours) and low protein binding (around 40 %).23,32 Although levosimendan is unlikely to pass across into milk, the active metabolites will, with the potential for drug accumulation in the infant. There is one case report in which levosimendan and the active metabolite (OR–1896) were measured in maternal plasma and breast milk. Levosimendan was not detected in milk or plasma; only the active metabolite was detected in milk (0.08–0.10 ng/ml).33 Because experience of its use is limited, breastfeeding while being exposed to levosimendan must proceed with extreme caution. Although exposure in the mother will be for 24 hours, because of the extended half-life of the active metabolites, the infant will have to be monitored for adverse effects for around a week after cessation of therapy for signs of hypotension, cardiac arrhythmias, hypokalemia, anaemia and gastrointestinal disturbances.23 Expressing and discarding for a short period is not recommended since peak maternal plasma levels will only be reached after 2 days,33 with a lag time for when peak concentrations will enter breast milk. This makes it difficult to calculate when such an abstinence period may be most beneficial, while adding complications for the mother.
Pharmacotherapy in Stable Heart Failure Secondary to PPCM
Blockade of the Rennin-angiotensin System
Angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in those who cannot tolerate an ACE inhibitor have been shown to improve survival in patients with heart failure through their effects on the renin-angiotensin-aldosterone system. They should be started once renal function and haemodynamic stability allow.4
Angiotensin-Converting Enzyme Inhibitors
In general, ACE inhibitors have poor bioavailability but are metabolised to active metabolites which have long half-lives.23,32 An exception to this is lisinopril, which does not have an active metabolite and has a shorter half-life than other ACE inhibitors.23 Therefore, although the pharmacokinetic profile of lisinopril appears favourable, there is no data to support its use during breastfeeding. For other ACE inhibitors, where evidence is available, only very small amounts of the parent drug and its active metabolite have been found in breast milk.35,36,37,38,39,40 Enalapril is often the preferred option since it has the most published data supporting its use. One study calculated the level of infant exposure to enalapril as 0.16 % of the maternal weight-adjusted dose.37 Enalapril can also be used therapeutically from birth.24 Other ACE inhibitors can be considered for use during breastfeeding with caution.
Angiotensin II Receptor Blockers
Angiotensin II receptor blockers (ARBs) have very high protein binding,23 so potentially there is less free drug available to pass into the breast milk compartment. Compared to other ARBs, losartan would be a preferable choice since it undergoes extensive first-pass metabolism, resulting in very low systemic bioavailability of around 33 %.41 In addition, the parent drug and its active metabolite have short half-lives (2 and 9 hours respectively).41 If choice allows for PPCM treatment, an ACE inhibitor would be preferable in terms of breastfeeding. However, if an ARB is required, breastfeeding can proceed with caution.
Angiotensin Receptor Neprilysin Inhibitor (ARNI)
The recently marketed angiotensin receptor neprilysin inhibitor (ARNI; sacubitril valsartan) complex has no published data regarding use during breastfeeding. Neither is there any evidence of use during breastfeeding of it component parts individually. After oral administration, the complex readily dissociates. The use of valsartan has been discussed above in the context of ARBs. Sacubitril has favourable pharmacokinetics, with a reasonably short half-life of the parent drug and metabolite (1.4 and 11.5 hours), high plasma protein binding (around 95 %), and relatively low oral bioavailability of both parent and metabolite (60 % and 23 %).42 Therefore, although evidence is lacking, its use should not pose significant risk to the breastfeeding infant. Use of the complex can therefore proceed with caution.
With any of the medicines used for blocking the renin-angiotensin system (ACE inhibitors, ARBs or ARNIs), the breastfed infant should be monitored for hypotension (especially in neonates), drowsiness, lethargy, and poor feeding and weight gain. In a newborn infant, renal function should be monitored24,25,41,42,43 In addition, the infant should be monitored for hyperkalemia if sacubitril valsartan is used.42
Mineralocorticoid receptor antagonists
Mineralocorticoid receptor antagonists (MRAs) are recommended in patients with heart failure and an ejection fraction of 35 % or less who remain symptomatic despite treatment with beta-blockers and ACE inhibitors. Eplerenone and spironolactone have both been shown to reduce mortality in heart failure.4
Spironolactone is the preferred choice during breastfeeding, although there is only one published case report for its use. This showed that from measured breast milk levels, only negligible amounts of the active metabolite canrenone (0.2 %) would be received by a breastfeeding infant.48 In addition, spironolactone can be used in full term neonates from birth.25 There is no data for the use of eplerenone during breastfeeding, but use would not be expected to pose any significant risk to the infant. Therefore breastfeeding can proceed with caution.
As with all diuretics, there is a concern that intense diuresis can affect the milk supply.27,28 Therefore, if high doses or combinations are used, the mother’s milk supply should be monitored by checking if the infant is putting on weight adequately. The infant should also be monitored for fluid loss, dehydration, and lethargy.25
Beta-blockers
Beta-blockers, given together with ACE inhibitors, are first-line therapy in patients with heart failure, reducing mortality through modulation of neurohormonal activation. Propranolol and metoprolol are usually the preferred choice of beta-blockers during breastfeeding because of available evidence, favourable pharmacokinetic profiles and use in the paediatric population.
Comparatively, there is a reasonable amount of evidence to support the use of metoprolol during breastfeeding with no adverse effects reported to date. Only small amounts have been found in breast milk (0.5–2 % of the maternal weight adjusted dose)44,45 and extremely low or undetectable infant serum levels.46,47 Other favourable characteristics include low bioavailability (around 50 %) and a short half-life (3–7 hours),23 as well as use in infants therapeutically from 1 month. Within the UK, metoprolol succinate is unlicensed and not readily available (unlike the tartrate salt) and, as such, bisoprolol, carvedilol or nebivolol are often preferred options regarding PPCM management.
Pharmacokinetically, bisoprolol does not have a favourable profile compared to other beta-blockers: it has very low protein binding (30 %), good oral bioavailability (90 %), a comparatively longer half-life (10–12 hours) and moderate lipid solubility.23 It has relatively high renal excretion (50 %),23 which can potentially lead to drug accumulation. However, there is one case report in which bisoprolol levels were measured in breast milk; the levels were undetectable, which is reassuring.49
Carvedilol has a more favourable pharmacokinetic profile during breastfeeding, with very high protein binding (98 %), low oral bioavailability (25 %) due to first-pass metabolism, and a shorter half-life (6 hours).23,50 However, it has high lipid solubility,23,50 suggesting that some passage into breast milk would be expected.
Nebivolol is metabolised via cytochrome P450 enzyme CYP2D6, which is subject to genetic polymorphism. This leads to wide inter-patient variability in peak plasma concentrations and elimination half-lives.23 For example, the peak plasma concentration of nebivolol is about 23 times higher in poor metabolisers than extensive metabolisers.51 Although the clinical significance of this remains uncertain,52 it becomes difficult to extrapolate how much nebivolol will transfer into breast milk, and consequently how an infant will respond. However, although nebivolol has high protein binding (98 %), it also has high lipid solubility.23 Overall, passage into breast milk would still be expected.
Some studies have potentially implicated beta-blockers in causing adverse effects in breastfed infants,53,54,55 although there have been no reports of this happening directly with bisoprolol, carvedilol or nebivolol.
Based on the available evidence, metoprolol succinate would be the choice in breastfeeding if available. If it is not available, carvedilol would be a preferred second choice option, although bisoprolol can be used with caution. The use of nebivolol should be avoided where possible, and should be considered only if other beta-blockers are not suitable.
Infants exposed to beta-blockers via breast milk should be monitored for drowsiness, lethargy, beta-blockade (especially bradycardia), and poor feeding and weight gain.
Other Pharmacological Treatments
Additional therapeutic options may need to be administered to those presenting with PPCM and can be given to patients who remain symptomatic despite optimum therapy with ACE inhibitors, beta-blockers and MRAs. The following considerations must be taken into account in mothers that are breastfeeding.
Digoxin
Although digoxin does not have a favourable pharmacokinetic profile, there is evidence to show that in practice, levels found in breast milk are very low. Oral doses of 0.25–0.75 mg daily give breast milk levels ranging from 0.41–1.9 μg/litre.56,57,58,59 In two of these studies, the infant serum level was measured and found to be undetectable in one infant whose mother was taking 0.25 mg daily for 10 days, and 0.2 μg/litre after a 0.75 mg daily dose.57,59 In one study, 11 women were given 0.5 mg intravenous digoxin as a single dose; from the data, it was extrapolated that an infant serum level of only 3 % of the maternal therapeutic dose would be achieved.60 There have been no adverse effects reported in infants exposed to digoxin via breast milk. In addition, digoxin can be used from birth therapeutically.24 Digoxin is therefore considered compatible with breastfeeding and no special monitoring is required.25
Hydralazine
Hydralazine is considered compatible with breastfeeding. There is a wealth of experience of its use in the postpartum period and it can be used therapeutically from birth.24 It has a favourable pharmacokinetic profile – low bioavailability (around 35 %), good protein binding (90 %), and a short half-life (maximum 8 hours).23 One study reported the milk level as 130 μg/l from a hydralazine dose of 30 mg three times daily; this was calculated as the infant being exposed to 13 μg hydralazine per feed.61 In another study of 10 lactating women taking hydralazine doses of between 10 mg and 40 mg, the average milk concentration was 240 nmol/l; the authors calculated that an infant would not be exposed to any more than 25 μg per day. In this study, serum levels were measured in two infants and shown to be only 4–7 % after direct infant therapeutic administration.62 Breastfeeding can therefore proceed with no special monitoring requirements.
Isosorbide Dinitrate
Isosorbide dinitrate has low molecular weight (236), low protein binding and variable bioavailability (10–90 %).23,32 There is concern that nitrates may cause nitrate intoxication in infants because they are susceptible to methaemaglobinaemia.25 This risk increases with high doses and long-term use24 but is a rare side-effect, even when infants are exposed to isosorbide dinitrate directly.32 Amounts in breast milk are unlikely to present an issue, even with long-term exposure.
Isosorbide dinitrate can therefore be used during breastfeeding with caution. Infants should be monitored for drowsiness, lethargy, and poor feeding and weight gain. Flushing is an unlikely side-effect in the infant when used at lower doses longer term.
Ivabradine
Its molecular weight (467) and protein binding (70 %)23,63 suggest that excretion into breast milk would be expected. However, ivabradine has low oral bioavailability (40 %) due to extensive first pass metabolism.62 Ivabradine has been identified as a teratogen in animal studies, although it is unclear what effect, if any, the potentially low levels in breast milk could have on the developing infant. Until more is known about ivabradine in breast milk, it must be used with extreme caution. The infant should be monitored for bradycardia and arrhythmias, and poor feeding and weight gain.5
Conclusion
Management of PPCM in a breastfeeding mother is challenging, given the complexities surrounding diagnosis and pharmacokinetic considerations in breastfeeding.
On-going data collection through an international registry aims to provide further details on disease presentation, comorbidities, diagnostic and therapeutic management of patients with PPCM, as well as information on their offspring;9 this will help to clarify efficacy and safety of pharmacotherapy in PPCM.
In any case, diagnosis should be made swiftly to allow prompt initiation of treatment. Pharmacokinetic considerations in breastfeeding should be reviewed for their suitability in breastfeeding to minimise the risk of harm to the infant, while optimising therapy for the mother.
Disclaimer
The risk assessments provided apply to maternal monotherapy and a healthy infant born at term. When mothers are on combinations of therapies, additive side-effect profiles need to be considered. Should the infant be premature or unwell, or if the mother taking extensive multiple medications, an individual risk assessment is required and specialist advice should be sought.
Clinical Perspective
- Documented evidence for the use of medicines during breastfeeding is largely very poor, if available at all, often leading to an incorrect decision that the mother should not breastfeed.
- Many factors affect infant drug exposure via breastmilk, including pharmacokinetics, pharmacodynamics, and the clinical condition of mother and infant. All of these factors should be used to assess the risk-benefit of continuing breastfeeding while taking medication.
- There are very few medicines in the management of PPCM which truly require a breastfeeding abstinence period.
- Currently those presenting with PPCM should be encouraged to breastfeed if they choose to do so, with any risks being managed with adequate infant monitoring.