In 1616, Sir William Harvey was the first person to describe the importance of right ventricular function.1 However, the right ventricle (RV) has received little attention in the past, with cardiology dealing mostly with the diseases of the left ventricle (LV) and their potential treatment. Since the early 1950s, however, the prognostic significance of RV function has been recognised in several conditions, primarily those involving the LV (e.g. chronic LV failure), the lungs and their vascular bed (e.g. pulmonary embolism, chronic pulmonary disease and pulmonary arterial hypertension) or the right-sided chambers (e.g. RV infarction, RV cardiomyopathies and congenital heart diseases).
Recent advances in imaging techniques have created new opportunities to study RV anatomy, physiology and pathophysiology, and contemporary research efforts have opened the doors to new treatment possibilities.2,3 Nevertheless, the treatment of RV failure remains challenging. This article aims to provide an overview of the pathophysiology, diagnosis and treatment of RV failure.
Anatomical and Physiological Particularities of the Right Ventricle
The RV is a unique chamber with distinct anatomy and physiology.4 It is coupled to systemic venous return and the pulmonary circulation. Since the pressure in the pulmonary circulation is generally much lower than it is in the systemic circulation, less muscle power is needed (a quarter of the LV stroke work).5 Therefore, the RV needs fewer muscle fibres and is much thinner than the LV, having about one-third of the thickness. Furthermore, venous return fluctuates, so the RV is much more compliant and is slightly larger (approximately 10–15%) than the LV, which allows it to accommodate large variations in venous return without altering end-diastolic pressure. Each systolic contraction leads to a primarily longitudinal shortening, whereas LV contraction is more circumferential.6 Notably, both ventricles share the septum, and up to 40% of the RV systolic function is dependent on septal contraction.7 During exercise, a 30–50% decrease in predicted VO2 max can be seen in healthy Fontan patients, which indirectly highlights the critical role of the RV for maintaining cardiac output.8
The RV consists of the inlet with the tricuspid valve, chordae tendineae, at least three papillary muscles, the trabeculated apex and the infundibulum (a muscular structure supporting the pulmonary valve leaflets). For imaging analysis, the RV is divided into four segments: the infundibulum, and the anterior, lateral and inferior wall.9
The right coronary artery, at least in most individuals, perfuses the RV free wall and the posterior third of the interventricular septum. The left anterior descending artery perfuses the apex and the anterior part of the septum. Unlike the LV, RV perfusion occurs both in systole and diastole and the collateral vessels of the RV are denser than those of the LV. However, because of its thinner wall and higher dependence on coronary perfusion pressure, RV perfusion is more vulnerable to an increase in RV size (intramural pressure) and systemic hypotension.10
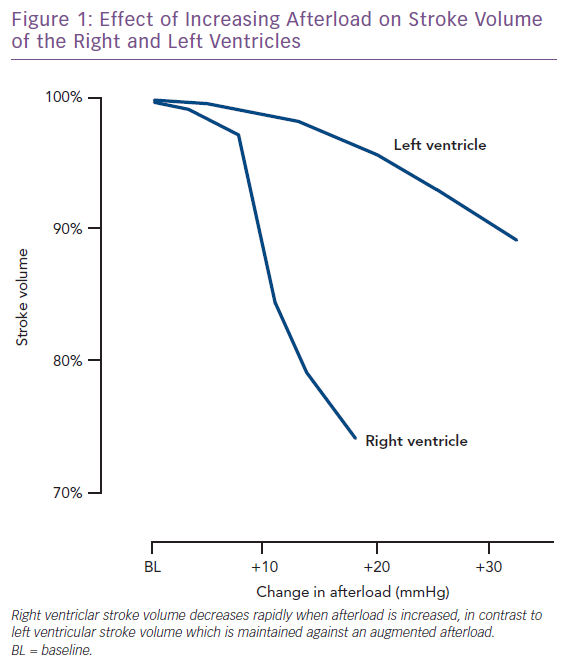
One of the main characteristics of the RV is its greater sensitivity to changes in afterload. Brisk increases in afterload are poorly tolerated and lead to RV dilatation to preserve stroke volume. One seminal work highlighted the response of the right and left ventricle to experimental increases in afterload. While an increase on the left side leads to only a slight decrease in stroke volume, the same increase in the RV results in a marked fall in stroke volume (Figure 1).
A further important characteristic is ventricular interdependence. Excessive RV volume loading is constrained by the pericardium and therefore results in compression and D-shaping of the LV Volume overload in the RV therefore indirectly leads to a decrease in LV stroke volume.
The anatomical and functional particularities of the RV have been reviewed in detail elsewhere.4
Causes and Pathophysiology of Right Ventricular Failure
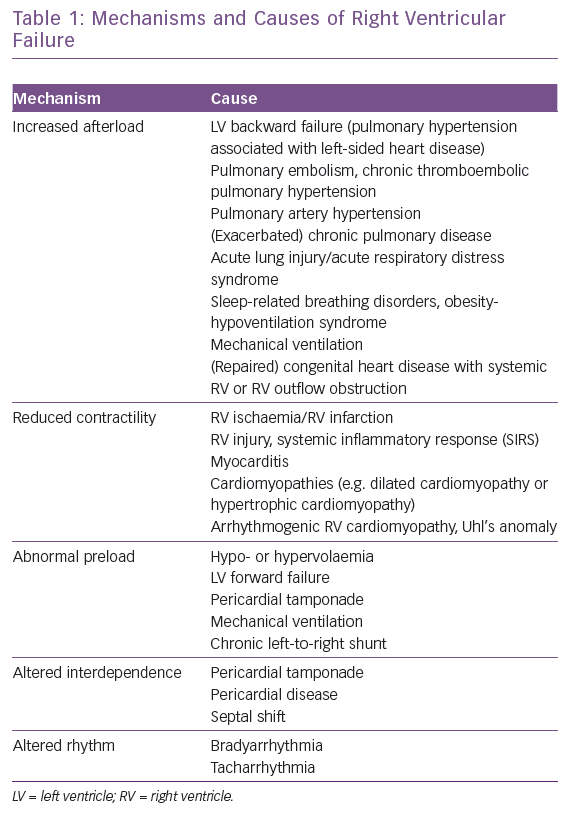
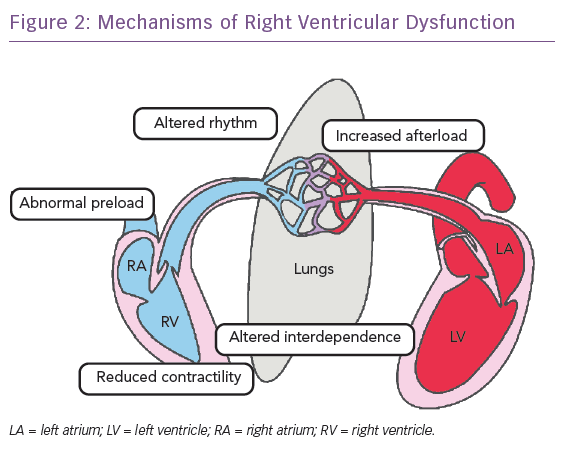
The normal RV function is an interplay between preload, contractility, afterload, ventricular interdependence and heart rhythm. Most cases of RV failure follow existing or new-onset cardiac or pulmonary diseases or a combination of both, which may increase RV afterload, reduce RV contractility, alter RV preload or ventricular interdependence or cause-related arrhythmias (Table 1 and Figure 2). To understand RV failure, it is crucial to assess these five components.
Right Ventricular Failure in Cardiac Disease
Increased afterload is the main pathophysiologic mechanism for RV failure of both pulmonary and cardiac origin. Indeed, the prevalence of left ventricular systolic or diastolic dysfunction and (post-capillary) pulmonary hypertension in patients with RV failure is particularly high, which corroborates the concept that the majority of RV failure is secondary to left-sided cardiac or pulmonary (vascular) diseases.11
Increased afterload is also a main cause of ventricular failure in patients with systemic RV (e.g. patients after atrial switch repair for complete transposition of the great arteries, with congenitally corrected transposition of the great arteries or after Fontan palliation) or with obstruction of the RV outflow tract. In patients with other forms of adult congenital heart disease (e.g. atrial septal defect with relevant left-to-right shunt or severe pulmonary regurgitation in repaired Fallot’s tetralogy), chronic volume overload may induce RV dilation and failure.
Cardiac diseases involving the right heart may primarily reduce RV contractility or, through reduced cardiac output, reduce RV preload, contributing to RV failure.
Virtually all myocardial diseases involving the left heart may affect the RV. These include myocardial ischaemia/infarction, myocarditis/septic cardiomyopathy, takotsubo cardiomyopathy, dilated cardiomyopathy, hypertrophic cardiomyopathy, cardiac amyloidosis and Chagas disease. Cardiomyopathies with primary involvement of the RV include arrhythmogenic RV cardiomyopathy (characterised by fibrofatty replacement of the RV myocardium), Uhl’s anomaly (which involves aplasia or hypoplasia of most of the RV myocardium), and Ebstein’s anomaly (defined as apical displacement of the septal and posterior tricuspid leaflets, which induces severe tricuspid regurgitation).
Pericardial diseases may alter RV preload and ventricular interdependence, while arrhythmia may aggravate RV dysfunction. Notably, iatrogenic RV failure through excessive volume loading or mechanical ventilation is frequently seen in critically ill patients, while ischaemic RV injury is sometimes seen after cardiac surgery. Finally, RV failure may be exacerbated in patients undergoing left ventricular assist device (LVAD) implantation, causing high morbidity and mortality and requiring temporary RV support. This topic has been reviewed extensively elsewhere.12
Right Ventricular Failure in Pulmonary Disease
RV failure as a consequence of lung disease is commonly described as cor pulmonale. These changes might occur dramatically – for example in fulminant pulmonary embolism – or might be due to longstanding respiratory disorders that result in chronic alterations of RV structure and function.
In the context of acute respiratory insufficiency in a previously healthy individual, impending RV failure is almost exclusively seen with massive pulmonary embolism. Of note, the elevation of pulmonary pressure following acute pulmonary embolism is observed only when more than half of the pulmonary vasculature is obstructed by thrombotic material.13 This is because distension and recruitment of additional pulmonary capillaries might decrease vascular resistance and compensate for circulatory changes.14 When thrombotic occlusion extends to more than 50% of the lung vessels and, in turn, pressure elevation occurs, the unconditioned RV can overcome a mean pulmonary arterial pressure of up to 40 mmHg.15 A higher afterload results in acute RV failure and obstructive shock. Conversely, if there is acute pulmonary embolism and the RV is exposed to higher pressure values and can tolerate it, a pre-existing elevation of pulmonary pressure (i.e. the presence of pulmonary hypertension) with an antecedent adaption of the RV must be assumed.
Many chronic lung diseases affect the pulmonary circulation and the right heart, but chronic obstructive pulmonary disease (COPD) is the most prevalent cause of respiratory insufficiency and cor pulmonale. COPD increases RV afterload by several mechanisms, including rarefaction of the vascular bed, hypercapnia and acidosis, pulmonary hyperinflation, airway resistance, endothelial dysfunction and hypoxia.16 Of these factors, hypoxia is arguably the most prominent driver of pulmonary hypertension and subsequent RV failure. Hypoxic pulmonary vasoconstriction (the Euler-Liljestrand effect) results in pulmonary pressure elevation and, when persistent, vascular remodelling and fixed pulmonary hypertension.17
The presence of pulmonary hypertension has long been considered as the conditio sine qua non for the development of a cor pulmonale.18 Recent data have challenged this assumption and suggested that, in patients with lung disease, structural alterations in cardiac myocytes predate the development of clinically manifested pulmonary hypertension.19 As such, cor pulmonale and failing RV syndrome in lung disease may be part of a disease spectrum rather than being distinct entities.20 With its impact on RV function, pulmonary hypertension – more than airflow limitation – is the strongest predictor of an adverse outcome and mortality in patients with lung disease.
Diagnosis of Right Ventricular Failure
Clinical Signs
The clinical signs of RV failure are mainly determined by backward failure causing systemic congestion. In severe forms, the right heart dilates and, through interventricular dependence, can compromise LV filling, reducing LV performance and causing forward failure (i.e. hypotension and hypoperfusion). Backward failure presents as elevated central venous pressure with distension of the jugular veins and may lead to organ dysfunction and peripheral oedema.21
The association between systemic congestion and renal, hepatic and gastrointestinal function in heart failure has been extensively studied.22 Elevated central venous pressure is the main determinant of impaired kidney function in acute heart failure.23,24 Hepatic dysfunction is also highly prevalent in acute heart failure; systemic congestion frequently presents with a cholestatic pattern, while hypoperfusion typically induces a sharp increase in circulating transaminases.25 Finally, systemic congestion may alter abdominal function, including reduced intestinal absorption and impaired intestinal barrier.26
ECG
The ECG in chronic RV failure often shows right axis deviation as a consequence of RV hypertrophy. Other ECG criteria are RS-ratio in lead V5 or V6 ≤1, SV5 or V 6≥7 mm, P-pulmonale or a combination of these. While the sensitivity of those criteria is quite low (18–43%), the specificity ranges from 83% to 95%.27 RV strain is sometimes seen in massive pulmonary embolism as an initial S deflection in I, an initial Q-deflection in III and T-Inversions in III (high specificity, low sensitivity), as well as in V1–V4.28 Moreover, RV failure is often accompanied by atrial flutter or AF.
Imaging
The primary working tool for imaging the (failing) RV is echocardiography. It should be emphasised that a comprehensive assessment of the anatomy and function of the right heart should include left heart function, pulmonary haemodynamics, the tricuspid valve and the right atrium. In most patients, transthoracic assessment by echocardiography is sufficient to describe RV morphology and function adequately.
However, because of the RV’s complex shape, echocardiography can only partially visualise it. Careful attention should be paid in obtaining an RV focused view from the apical four-chamber view with rotation of the transducer to obtain the maximal plane.8 Other views, such as the short axis and RVOT view, add anatomical and functional information. The measurements of RV function that are most frequently used and easiest to perform are fractional area change, tricuspid annular plane systolic excursion (TAPSE), pulsed tissue Doppler S’ or RV index of myocardial performance (RIMP). However, RIMP is rarely used and cumbersome to calculate.29,30
Guidelines recommend a comprehensive approach and using a combination of these measurements to assess RV function as none of them alone can adequately describe RV function in different scenarios.29 Moreover, these measurements are all somewhat load dependent and therefore subject to physiologic variation. Newer imaging techniques, such as 3D-echocardiography and strain imaging, have proven to be useful and accurate imaging modalities but have limitations because they depend on good image quality and lack validation in larger cohorts.31,32
Cardiac MRI has become the standard reference method for right heart acquisition as it is capable of visualising anatomy, quantifying function and calculating flow. In addition, it is useful in cases where image quality by echocardiography is limited. Moreover, it can provide advanced imaging with tissue characterisation, which is useful in different cardiomyopathies, such as arrhythmogenic RV cardiomyopathy, storage disease and cardiac tumours. Limitations are mainly due to the thinness of the RV wall, which can make it challenging to differentiate it from surrounding tissues.9 In addition, pacemakers or pacemaker leads may interfere with image acquisition during MRI and lead to artefacts that impair visualisation of the RV walls.
Cardiac CT and nuclear imaging play a minor role although cardiac CT can help to visualise anatomy when MRI is not feasible. There are concerns regarding radiation exposure from both nuclear imaging and dynamic imaging by CT angiography.
Medical Treatment of Acute Right Ventricular Failure
The Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology recently published a comprehensive statement on the management of acute RV failure.33 The triage and initial evaluation of patients presenting with acute RV failure aim to assess clinical severity and identify the cause(s) of RV failure, with a focus on those requiring specific treatment. Management of acute RV failure requires not only an understanding of the anatomical and physiological particularities of the RV but also rapid identification and treatment of the underlying causes and related pathophysiological disorders (see above).
In this context, echocardiography and other imaging modalities are frequently essential to identify the cause of RV failure and guide treatment. In patients presenting with severe RV failure, rapid initiation of treatment to restore haemodynamic stability is essential to prevent significant, potentially irreversible end-organ damage.
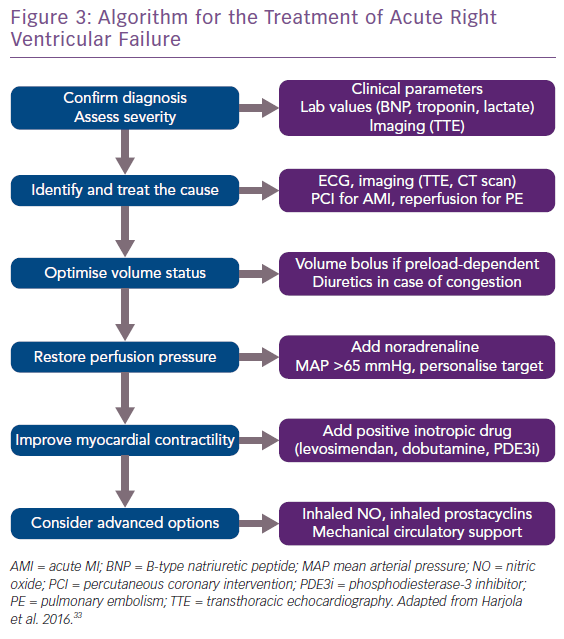
Acute treatment consists of four elements: volume optimisation; restoration of perfusion pressure; improvement of myocardial contractility; and advanced options (Figure 3).
Volume Optimisation
A common misconception is that RV failure should consistently be treated with volume supplementation. Conversely, while the RV might physiologically be able to accommodate large variations in preload and some patients with RV failure are preload dependent, a large proportion of RV failure is caused, associated with or aggravated by RV volume overload. In such cases, volume loading has the potential to overdistend the RV and thereby increase wall tension, decrease contractility, aggravate tricuspid regurgitation, increase ventricular interdependence, impair LV filling and, ultimately, reduce systemic cardiac output and exacerbate organ dysfunction.24,33,34 In patients with RV failure and signs of venous congestion, diuretics are often the first option to optimise volume status.
Notably, in patients with massive renal congestion due to severe RV dysfunction and/or severe tricuspid regurgitation, sufficient renal perfusion pressure (i.e. mean arterial pressure minus central venous pressure) and an adequate diuretics plasma concentration are crucial to achieving the desired effect. Furthermore, since most of the effect of IV loop diuretics occurs within the first hours – with sodium excretion returning to baseline within 6–8 hours – 3–4 daily doses or continuous infusion are required to maintain the decongestive effect.35 In the context of RV failure, early evaluation of the diuretic response (by measuring urine output or post-diuretic spot urinary sodium content) to identify patients with an inadequate diuretic response is even more important than it is in other forms of acute heart failure. If decongestion is insufficient, rapid intensification of loop diuretic dose, starting a sequential nephron blockade (combining diuretics with a different mode of action) or the use of renal replacement therapy/ultrafiltration should be considered.
In the absence of elevated filling pressure, cautious volume loading guided by central venous pressure monitoring may be appropriate.33
Restoration of Perfusion Pressure
Vasopressors are primarily indicated to restore arterial blood pressure and improve organ perfusion. Noradrenaline can restore systemic haemodynamics without increasing RV afterload (i.e. there is no effect on pulmonary vascular resistance).36 Restoration of coronary perfusion pressure by vasopressors is a mainstay of therapy since the failing RV dealing with volume and/or pressure overload is particularly susceptible to ischaemic injury. Furthermore, vasopressors restore cerebral, renal and hepato-splanchnic perfusion pressures. Clinical data suggest that targeting a mean arterial pressure (MAP) of 65 mmHg may be reasonable. However, MAP alone should not be used as a surrogate measure of organ perfusion pressure, especially in patients with RV failure and severe tricuspid regurgitation with massively elevated central venous pressure. Organ-specific perfusion pressure targets include 50–70 mmHg for the brain, 65 mmHg for renal perfusion and >50 mmHg for hepato-splanchnic flow.37 Therefore, the MAP targets should be personalised based on the measures of organ function and tissue perfusion.
Improvement of Myocardial Contractility
Dobutamine, levosimendan and phosphodiesterase III inhibitors improve contractility and increase cardiac output and are indicated in patients with severe RV failure causing cardiogenic shock despite treatment with vasopressors.33 Levosimendan and phosphodiesterase III inhibitors may favourably affect the ventricular-arterial coupling by combining RV inotropy and pulmonary vasodilation and might be preferentially indicated in patients with pulmonary hypertension caused by left heart disease.24,38 The use of epinephrine is not recommended.39–41
Advanced Options
In patients with pre-capillary pulmonary hypertension, therapy should be driven by treatment of the underlying disease. Long-term oxygen therapy in hypoxic patients might stabilise pulmonary hypertension despite continued progression of lung disease, whereas supplementary oxygen in patients without hypoxia or moderate desaturation is not beneficial.42,43 The role of pulmonary vasodilators is highly controversial. Intravenous prostacyclin analogues effectively reduce RV afterload, but may aggravate systemic hypotension. Alternatively, inhaled nitric oxide or inhaled prostacyclin may be considered.33 These agents should be used only in an appropriate setting (specialised units) and in selected patients because of the risk of an increase in ventilation/perfusion mismatch and subsequent clinical deterioration. Notably, long-term therapy with phosphodiesterase-5 inhibitors, endothelin receptor antagonists, guanylate cyclase stimulators, prostacyclin analogues and prostacyclin receptor agonists are not recommended for the treatment of pulmonary hypertension due to left heart disease, which is the most prevalent cause of RV dysfunction.
In patients with refractory RV failure despite treatment with vasopressors and inotropes, advanced therapeutic options including fibrinolysis for pulmonary embolism or mechanical circulatory support should be considered (see below).
In the absence of long-term therapeutic options, palliation and supportive care should be offered to patients and relatives.44
Mechanical Circulatory Support for Advanced Right Ventricular Failure
Mechanical circulatory support with RV assist devices (RVADs) should be considered when RV failure persists despite treatment with vasopressors and inotropes (Figure 3). Because reversibility of severe RV failure is more likely to be possible and more rapid than LV failure of similar magnitudes, temporary RVADs (t-RVADs) can be a valuable therapeutic option for many patients.45 The often-reported poor survival rates of RVAD recipients should not discourage the appropriate use of t-RVADs, because patient mortality depends mainly on the primary cause of RV failure, the severity of end-organ dysfunction and the timing of RVAD implantation, and much less on adverse events and complications related to RVAD implantation, mechanical support or removal (selection bias).12 The most important determinants of success are optimal patient selection (according to age, comorbidities, RV dysfunction aetiology and reversibility potential) and optimal timing of implantation to avoid significant, potentially irreversible end-organ injury. For that reason, these patients require close haemodynamic and laboratory monitoring, with particular attention to liver and kidney function, and early transfer to a centre with experience in RVAD implantation in case of persistent haemodynamic instability.
Choosing the most appropriate device also maximises the success of mechanical circulatory support. First, the strategy (e.g. bridge to recovery, bridge to bridge – i.e. LVAD/biventricular VAD/total artificial heart – or bridge to transplant) should be defined. Second, the need for an oxygenator should be anticipated because it may influence device selection. Third, the function of both the LV and RV should be carefully assessed to predict the need for isolated RV support or biventricular support (either durable or temporary). In addition to device characteristics, it is crucial to consider local expertise and availability.
For short-term support, several t-RVADs, both percutaneous and surgical devices, are available.
Percutaneous t-RVADs allow early initiation of support without the need for surgery. They are approved for a shorter period of time, and can sustain a lower flow than surgical devices. They are categorised according to their mechanism of action as either ‘direct RV bypass’, such as the Impella RP (Abiomed) and TandemHeart RVADs (TandemLife) or ‘indirect RV bypass’ systems, such as venous-arterial ECMO (VA ECMO).46 Impella RP is a microaxial flow 22 Fr catheter, approved for 14 days’ use, that delivers blood (at a rate of up to 4 l/min) from the RA into the pulmonary artery (PA).47 The TandemHeart RVAD uses an extracorporeal centrifugal flow pump and two venous cannulas or a single cannula with two lumens (Protek Duo cannula) to deliver blood from the RA to the PA with the additional possibility of oxygenating the blood.48 Directly bypassing the RV function reduces RA pressure, raises mean PA pressure and increases left ventricular preload, while the left ventricular afterload remains unchanged. The VA ECMO is the less expensive device. It bypasses the RV indirectly, displacing venous blood from the RA across an oxygenator into the peripheral arterial circulation. It induces a decrease in RA and PA pressure and LV preload but increases LV afterload if not cannulated centrally via surgical access.49
Surgical t-RVADs require an open sternotomy or thoracotomy for direct RA and PA cannulation and their connection to an extracorporeal centrifugal flow pump. They allow more extended and greater support in terms of flow at the cost of an invasive implantation and removal. However, more recently developed surgically implantable short-term extracorporeal CF-RVADs can be removed without reoperation; that is, the CentriMag system (Chalice Medical), which allows 30 days of support with up to 10 l/min blood flow).50
For more durable, long-term support, isolated pulsatile RVADs, surgically deployed rotary-flow RVADs and biventricular support with pulsatile VADs or total artificial heart replacement are potential options; however, the majority of patients using these are required to remain in the hospital under close surveillance. This is one reason why the use of durable isolated right ventricular assist devices (e.g. LVADs in the RV position) to support isolated RV or biventricular failure has been evaluated.51–54 A significant limitation of this approach is that LVADs are designed to operate in the systemic circulation with higher resistance and, where pulmonary resistance is low, they are more prone to complications, such as repetitive suction events. Because of the many technical limitations of durable RV support and because RV function may not recover, cardiac transplantation remains the only successful long-term treatment.
Arrhythmic Aspects of Right Ventricular Failure
Cardiac rhythm plays an important yet often underestimated role in RV function. One one hand, the failing RV, specifically if experiencing an increased afterload, such as in pulmonary hypertension, is highly dependent on a regular heart rate to function adequately, as its contractile reserve is very limited.55 The need for a constant sympathetic drive to maintain cardiac output may be one reason why beta-blocker therapy is not effective in right heart failure.56
On the other hand, RV pressure overload, an integral part in the pathophysiology of RV failure, is often associated with supraventricular arrhythmias, such as atrial fibrillation, atrial flutter or (multi-)focal atrial tachycardia, all of which negatively affect RV filling and thereby contribute to the vicious cycle of aggravating RV failure, eventually culminating in cardiogenic shock. Therefore, in addition to careful volume management to optimise RV preload and RV wall stress, prompt rhythm control of supraventricular tachyarrhythmias is central.
Importantly, diastolic filling of the failing RV depends on atrial contraction (‘atrial kick’) and atrioventricular synchrony.57,58 Therefore, rate control alone is generally insufficient to restore haemodynamic stability.59 In the acute setting, prompt electrical cardioversion (ECV) is the treatment of choice to restore sinus rhythm, although ECV efficacy in restoring and maintaining sinus rhythm may be reduced in critically ill patients.58,60,61
Retrospective analyses in patients with pulmonary hypertension indicate that maintaining sinus rhythm is associated with a reduction in clinical deterioration.62 Since beta-blockers and calcium channel antagonists may hamper RV contractility because of their negative inotropic effect, amiodarone should be used if antiarrhythmic medical therapy to maintain sinus rhythm is warranted. Class Ic antiarrhythmics, as well as sotalol and dronedarone, should not be used in structural heart disease. If medical therapy fails, AV synchronous pacing, with either the patient’s indwelling device or transvenous pacing wires may be considered.2
The interplay of the right and left ventricles, sharing their interventricular septum and competing for the limited space within the pericardium, leads to ventricular interdependence.33 Consequently, the loss of synchronous ventricular contraction is associated with a significant deterioration of RV contractile force. Notably, cardiac resynchronisation therapy (CRT) is standard care in patients with heart failure with reduced LV ejection fraction (HFrEF) and a wide QRS complex (>130 ms), and serves to resynchronise LV contraction. Many patients with HFrEF also have reduced RV function, either as a consequence of increased RV afterload (post-capillary pulmonary hypertension) or secondary to a cardiomyopathy affecting both ventricles.33
Because of anatomical and technical obstacles (difficult reliable assessment of RV function and the absence of an epicardial RV venous system for safe lead placement) and as RV function has gained attention only in recent years, very limited data on isolated right cardiac resynchronisation exist. However, though scarce, there are data on the interplay of LV resynchronisation and RV function. Indeed, reverse LV remodelling is associated with reduced RV afterload.63
Not surprisingly, several observational studies have demonstrated significant improvement in RV function after CRT.64 Similarly, CRT was associated with significant improvement of RV fractional area change in a post-hoc analysis of the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy trial (MADIT-CRT).65 However, no such effect was observed in a post-hoc analyses of the REsyncronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) and Cardiac Resynchronization-Heart Failure (CARE-HF) trials, which used TAPSE to assess RV function.66,67 Of note, TAPSE is mainly influenced by longitudinal movement of the RV free wall and may underestimate the contribution of the interventricular septum and outflow tract. Importantly, both observational studies and post-hoc analyses of randomised clinical trials may serve only to generate hypotheses and do not allow reliable conclusions. The value of left cardiac resynchronisation in isolated RV failure is unknown.
Similarly, data on the prevalence of sudden cardiac death (SCD) in isolated RV failure are scarce, at best. It is therefore not surprising that current guidelines do not recommend ICD therapy for primary prevention of SCD in patients with isolated RV failure.68 An exemption is patients with arrhythmogenic RV cardiomyopathy, in whom, if certain risk factors for SCD are present, an ICD for primary prevention of SCD may be considered (IIb; level of evidence C). In cases of secondary prevention, on the other hand, after documented haemodynamically relevant ventricular tachycardia or following survived SCD, and after secondary causes have been excluded, ICD therapy is recommended, regardless of right or left ventricular function.
Conclusion
The assessment of RV failure should consider the anatomical and physiological particularities of the RV and include appropriate imaging techniques to understand the underlying pathophysiological mechanisms.
Treatment should include rapid optimisation of volume status, restoration of perfusion pressure, and improvement of myocardial contractility and rhythm and, in case of refractory RV failure, mechanical circulatory support.