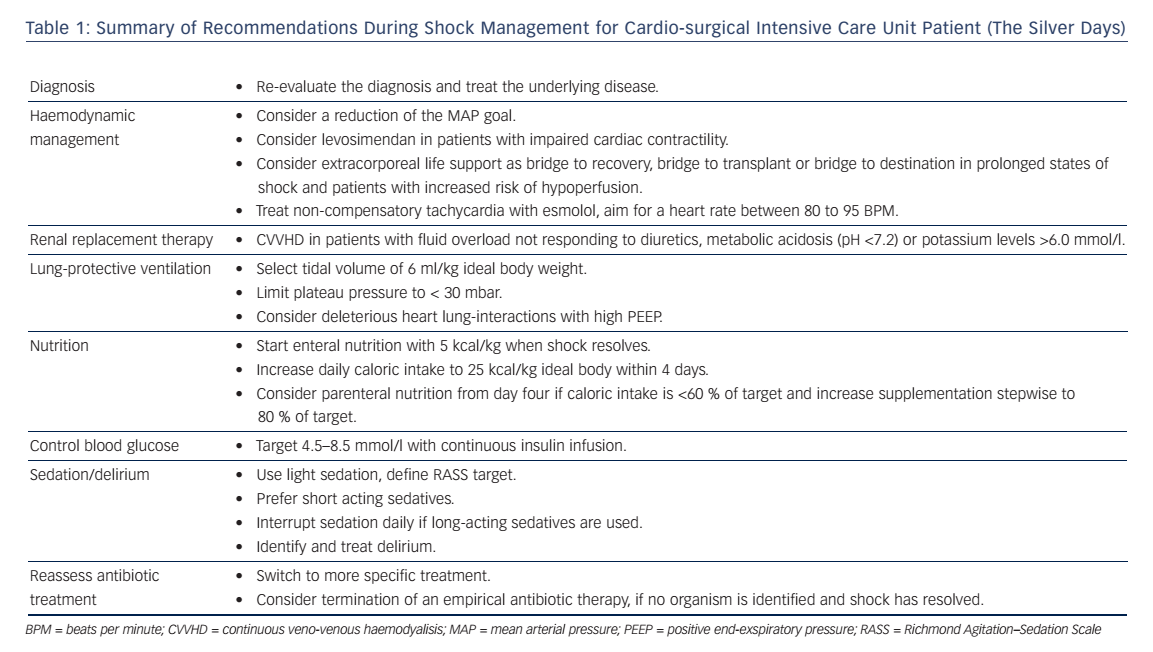
Shock in cardio-surgical intensive care unit (ICU) patients is a serious condition associated with a high morbidity and mortality.1,2 Prompt identification of the underlying condition and timely therapeutic interventions are key to reverse the shock state and to improve the patients’ outcome. Hence, the management during the first 6 hours is of paramount importance. This time period is also referred to as “the golden hours”. Ideally, a correct diagnosis is established allowing specific treatments. The authors have previously described a state-ofthe-art diagnostic work-up and discussed how to optimise preload, vascular tone, contractility, heart rate and oxygen delivery during this phase.3 Ideally, shock can be reversed during this initial period, however some patients might have developed multiple organ dysfunction that persists beyond the first 6 hours despite the early haemodynamic treatment goals been accomplished.4 This period, also referred to as “the silver days”, is the focus of this review. The authors discuss the management of organ dysfunction in critically ill patients after cardiac surgery. The following recommendations (summarised in Table 1) are not exclusive, rather they highlight some important considerations to be made while treating these patients after the initial resuscitation phase.
Methods
For this narrative review, a search of the PubMed database and a review of bibliographies from selected articles were performed to identify original data relating to this topic. Key words used for the search were, among others, “haemodynamic management” “vasopressors”, “levosimendan”, inotropes”, inotropic therapy”, “nutrition”, “sedation”, “ventilation”, “weaning from mechanical ventilation”, “delirium” and “procalcitonin”. National and international guidelines were reviewed and integrated, e.g. the “Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure” of the European Society of Cardiology (ESC)5 and the “Consensus on circulatory shock and haemodynamic monitoring“ from the Task force of the Society of Intensive Care Medicine (ESICM).6
Articles were scrutinised regarding their study design, population evaluated, interventions, outcomes and limitations. Finally, personal recommendations were included and highlighted as such to give a comprehensive overview on this topic.
Optimise Haemodynamics
Reduce Vasopressor Load
The optimal mean arterial blood pressure (MAP) under vasopressor therapy is still under investigation. For patients with septic shock, no benefit was found after increasing the MAP by use of vasopressors above 65–70 mmHg.7 Moreover, higher vasopressor loads were associated with higher mortality.8 However Asfar et al. showed that targeting a MAP of 80–85 mmHg in patients with chronic arterial hypertension reduced the incidence of renal replacement therapy.7 Therefore, the authors follow the recommendations of the 2014 consensus report of the ESICM6 and target an individualised blood pressure rather than fixed MAP-goals after the first phase of lifesaving measures. In patients that remain anuric despite a MAP of 70 mmHg, the blood pressure target is reduced. In patients with critical vascular stenosis or right heart failure, the MAP is not reduced <65 mmHg. In all others, MAP targets are continuously reduced and sometimes tolerated as low as 50 mmHg in order to reduce the amount of vasopressors necessary.
Consider Alternative Inotropes
Beta-adrenergic drugs have been associated with considerable risks including adverse effects on metabolism, bacterial growth and alterations of the innate immune response.9–13 Also, the amount and duration of catecholamines is independently associated with adverse cardiac events such as tachyarrhythmia and prolonged elevated heart rate.14 Hence, efforts are necessary to limit the use of these drugs as much as possible. One alternative is the inodilator levosimendan. It increases the troponin C affinity for Ca2+, which results in strengthening of the myocardial contraction without increasing oxygen demand.15 Also, it has vasodilatorproperties via activation of ATP-dependent potassium channels.16 The use of Levosimendan in cardiogenic shock is controversial because patients with cardiogenic shock have been excluded in safety studies as levosimendan causes vasodilation.17 It is recommended by the 2013 ESC guidelines on heart failure in cardiogenic shock and second line treatment for low output heart failure if the effect of beta blockage is thought to be the reason for hypoperfusion.5 The Levosimendan Infusion versus Dobutamine (LIDO) study showed a survival benefit of levosimendan after 6 months.16,18 The Survival Of Patients With Acute Heart Failure In Need Of Intravenous Inotropic Support (SURVIVE) study found an advantage in patients with decompensated chronic heart failure previously treated with beta blockers.17,19,20 Another study showed the combination of levosimendan and dobutamine to be more effective compared to dobutamine alone.21 In cardiac surgery, levosimendan has shown to produce a dose dependent effect on stroke volume, also when given preoperatively in patient at risk, and to shorten length of ICU stay.22 It is the only inotrope which might decrease the mortality after cardiac surgery.23
The authors use levosimendan for persisting heart failure after shock resolution, weaning failure from inotropic therapy, or weaning from extracorporeal life support (ECLS) with a dose of 0.1–0.2 mcg/kg/min without a bolus, if systolic blood pressure is >100 mmHg with low dose vasopressors (noradrenaline <0.1 mcg/kg/min). Experimental and clinical studies also suggest an improvement of cardiac function with levosimendan in patients with septic shock.17,24
Evaluate Mechanical Support
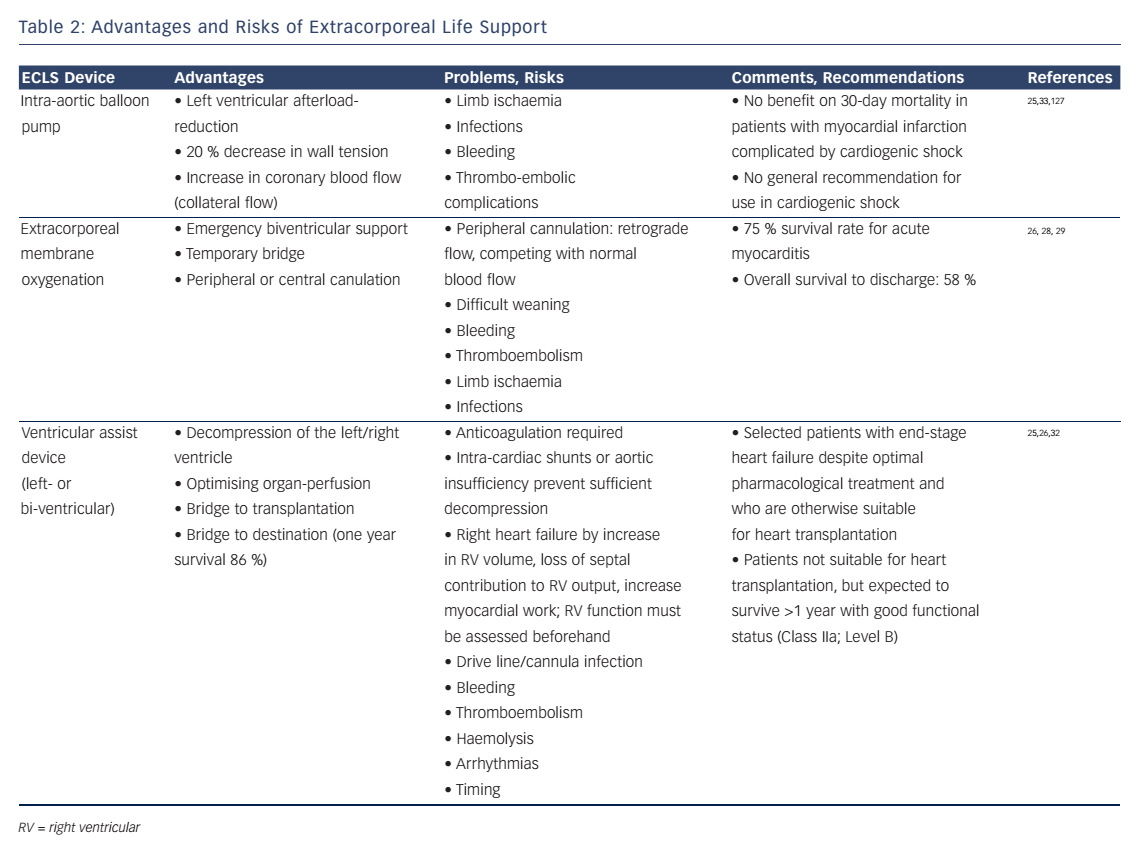
In situations with increasing inotrope requirements or inadequate oxygen delivery despite high doses of inotropes, mechanic circulatory support must be evaluated.25 ECLS with an extracorporeal membrane oxygenator can be established quickly in experienced hands and offers an opportunity for temporary haemodynamic support.26 This might give time for decision-making (bridge to decision) or for the ventricle to recover (bridge to recovery). The ESC guidelines give IIb and IIa recommendations for short-term mechanical support as a bridge to decision and bridge to recovery, respectively.5 In patients with multiple organ dysfunctions mortality rates may be excessively high, prohibiting the use of ECLS in this particular patient population.27 Recently, the concept of awake ECLS has been introduced.28 The benefits of having patients on ECLS without mechanical ventilation include the possibility to assess the patients’ cognitive functions. It also allows interaction with the patient and the possibility to inquire his will, particularly in view of future therapeutic options (ventricular assist device, transplantation). As positive pressure ventilation and sedation can be avoided, patients awake on ECLS have a better haemodynamic stability.
In selected patients with persisting cardiogenic shock under optimised pharmacologic therapy and/or weaning failure from ECLS, commercially available left- or biventricular assist devices (LVAD or BVAD) will provide sufficient organ perfusion for everyday life. These devices may be used in end-stage heart failure patients before heart transplantation (bridge to transplantation).26 Mechanical support will allow early rehabilitation leading to improved nutritional state, muscle strength and physical performance status. According to the ESC heart-failure guidelines, LVADs are a IIa indication for long-term use when transplantation is not possible (bridge to destination).5
Increased cardiac output by a LVAD may enlarge right ventricular (RV) volume load and cause RV-failure. Therefore, RV function must be assessed before considering the implantation of a LVAD.25 Surely the decision should be made in a multidisciplinary approach by experts due to the invasive nature of these devices. Complications may be related to the mandatory use of anticoagulation (bleeding, thromboembolism), mechanical shear stress on cellular blood components (haemolysis) and long-term infections of the implanted materials (drive line, cannulas, device).25,26,29–33 A summary of the advantages and risks of ECLS is provided in Table 2.
Control Heart Rate
Adrenergic stress might induce inflammation and contribute to the pathogenesis of organ dysfunction.12,34 Hence, the use of beta-adrenergic drugs must be limited to a minimum. Taking this concept to the next level, the theoretical benefits of beta-blockers during critical illness have been discussed.35 Recently, Morelli et al. tested the effects of esmolol in septic patients.36 He included high-risk patients, who required noradrenaline and had a heart rate ≥95 beats per minute (BPM) despite 24 hours of haemodynamic optimisation (MAP ≥65 mmHg, pulmonary artery occlusion pressure ≥ 12 mmHg, SvO2 ≥65 %). Esmolol was started at a dose of 0.5 mg/min and increased by 0.5–1.0 mg/min increments at 20-minute intervals to an upper dose limit of 30 mg/min. The goal was a heart rate reduction to a rate between 80 to 94 BPM. If mixed venous oxygen saturation (SmvO2) decreased below 65 % and/or arterial lactate concentrations increased despite appropriate oxygenation (SaO2 ≥95 %) and a haemoglobin concentration ≥8 g/dl, levosimendan was administered at a dose of 0.05–0.2 mcg/kg/min for 24 hours. In their cohort study from 2013, the authors showed improvements in stroke volume, a decrease in norepinephrine requirement and a reduction of mortality.36 If these results are confirmed in future multicentre trials, this concept might revolutionise current practice in sepsis management. Potentially, this new concept might be usefully expanded to other critical ill patients with non-compensatory tachycardia.
A reduction in heart rate will allow a better ventricular filling in patients with diastolic myocardial dysfunction, resulting in an increase in stroke volume. The same is true for patients with supraventricular arrhythmias and atrial fibrillation (AF) with a high ventricular response rate.37 Additionally, a reduction in heart rate will reduce myocardial oxygen expenditure, hence inducing cardio-protection. However, some patients will have a high heart rate in order to compensate for a low stroke volume and/or an insufficient oxygen delivery. In these patients, prolonged heart rate reduction will result in haemodynamic collapse and eventually death. Hence, short-acting drugs should be used in patients in which it is unclear whether tachycardia is compensatory or not. In such situations, the authors use esmolol, a selective beta1- blocker with a short half-life of 9 minutes, as an intravenous bolus (10–20 mg-wise up to 1 mg/kg), followed by a continuous infusion of 0.05 mg/kg/min. The infusion rate can be increased every 30 minutes if needed. Of note, the negative inotropic effects of esmolol must be balanced against the potential benefits, and close haemodynamic monitoring including echocardiography is mandatory in unstable patients treated with beta-blockers.
Start Fluid Weaning
Fluid resuscitation is one of the corner stones of shock therapy to restore tissue perfusion.38 However, it was demonstrated that a liberal fluid regime increased mortality and morbidity in a diverse group of patients.39 Targeting a central venous pressure (CVP) between 8–12 mmHg seemed to impair the microcirculation, was a risk factor for acute kidney injury (AKI) and increased mortality.39 The underlying mechanism seems to be a consecutive reduction in renal blood flow and glomerular filtration rate by a high venous pressure.39,40 Of note, there is no good correlation between CVP and fluid responsiveness41–43 and therefore other preload variables should be used.44
Since fluid overload leads to increased morbidity in acute respiratory distress syndrome,45 pancreatitis46 and sepsis,47 the authors recommend a conservative fluid regime with fluids being administered preferably after assessing responsiveness.6 As soon as the patient has stabilised and noradrenaline requirements are below 10 mcg/min, fluid weaning is started. In haemodynamic stabile patients with AKI, protocolled renal replacement therapy (RRT) allows large negative fluid balances.48 Net fluid removal usually causes improvements in lung function, therefore leading to a reduction in ventilation days. Reduction of oedema in the bowel and the extremities will facilitate nutrition and mobilisation of the patient.
Ventilate Safely
The authors use also the concept of lung-protective ventilation derived from acute respiratory distress syndrome (ARDS) patients for ICU patients with normal lungs. A tidal volume of ≤6 ml/kg should be chosen with plateau pressure of <30 mbar. Positive end-expiratory pressures (PEEP) are increased with increasing oxygen requirements. However, high PEEP levels increase RV afterload and might cause RV dysfunction.49 These heart-lung interactions might reduce RV stroke volume and consequently cardiac output, thereby decreasing oxygen delivery to the periphery. In case of further aggravation of hypoxaemia under increased PEEP, a persisting foramen ovale should be excluded. Hypercarbia causes pulmonary vasoconstriction and increases RV afterload, thus normoventilation should be targeted if possible in patients with pulmonary artery hypertension and/or RV dysfunction. Another risk of invasive ventilation is ventilator associated pneumonia (VAP), which was defined by the following criteria during a time frame of 48 hours after intubation:50,51
- new or progressive pulmonary infiltrates;
- fever;
- leucocytosis;
- purulent secretion;
- reduction of the PaO2:FiO2 ratio by ≥ 15 %.
In the American Thoracic Society definition from 2005, VAP is defined as pneumonia occuring 48–72 hours after intubation.52 However, diagnosis of VAP remains difficult as criteria are nonspecific and symptoms overlap as in conditions like sepsis, ARDS or atelectasis.50 Radiographic signs are neither specific nor sensitive. In a study comparing chest X-ray findings in autopsy proven pneumonia, no sign had a diagnostic efficiency greater than 68 % and positive air bronchogramms predicted 65 % of pneumonias.53 Thus, a diagnosis of VAP is made by a combination of clinical signs, radiographic findings and, importantly, microbiological cultures.54,55
To reduce the incidence of VAP our hospital implemented the following bundle:56
- Strict hand disinfection,
- oral hygiene with application of chlorhexidin,
- elevation of upper body >30° (if possible from a haemodynamic point of view),
- defined weaning and sedation protocols,
- continous subglottical suction,
- cuffpressure-control,
- daily reevaluation of gastric ulcer prophylaxis,
- Routine change of ventilator circuits and filters.
Manage Acute Renal Dysfunction
Understand Renal Dysfunction
Acute kidney injury (AKI) has a high incidence in patients after cardiac surgery varying between 1 and 30 %.57–61 The acute kidney injury network (AKIN) and risk, injury, failure, loss (RIFLE) criteria of AKI are accurate and early predictive of mortality with creatinine levels being the most useful marker.57,59,62,63 The pathophysiology is complex and involves pre-, intra- and post-operative risk factors.57,64 Most vulnerable is the patient with pre-existing renal dysfunction prior to surgery.57,59 Nephrotoxins such as contrast agents and antibiotics, impaired cardiac output and poor renal perfusion, inflammation caused by surgical trauma, cardio-pulmonary-bypass and sepsis are further factors contributing to AKI.59,65 No preventive pharmacological therapy has been proven to be effective although studies indicate a possible benefit of statins pre-operatively.57,66–68 If possible, cardiac surgery should be performed ≥24 hours after coronary angiography to reduce the risk of AKI.69 Patients who need renal replacement therapy have a 27 fold increased mortalitiy.70
Start Renal Replacement Therapy
The ideal time point of RRT-initiation remains unclear since the benefits of RRT must outweigh the risks of RRT61,71–74 In their cardiosurgical ICU the authors start RRT with continuous veno-venous haemodialysis (CVVHD), if the patient has metabolic acidosis (pH <7.2), increasing potassium levels (>6.0 mmol/l), complications of azotaemia (blood urea >20 mmol/l) such as encephalopathy or fluid overload not responding to diuretics.
The current Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend a dose of 20–25 ml/kg/hour of effluent flow in patients with AKI emphasising the need to individualise the patients dose by assessing volume status, acid-base status and electrolyte disturbances.62 Anticoagulation is required to prevent membrane clotting and dysfunction. Regional anticoagulation with citrate has the advantage over heparin75 to reduce the bleeding risk in post-operative patients after cardiac surgery, as heparin anticoagulation can be complicated by platelet- and red blood cell-consumption.75 However, impaired cellular aerobic metabolism (Krebs cycle) puts the patients at risk for insufficient citrate metabolism and citrate accumulation. In order to avoid this risk, the authors accept post-filter levels of calcium [ionised] as high as 0.5 mmol/l and limit the amount of citrate used (blood flow as low as 100 ml/minute, citrate concentration fix 3 mmol/l). The calcium quotient (total calcium [albumin corrected] divided by calcium [ionised] is measured daily. A value of ≥2.5 points to citrate accumulation and should prompt either a reduction of citrate load or a switch to alternative anticoagulation strategies.
Interrupt Sedation Daily
After cardiac surgery most patients arrive in the ICU sedated and with some neuromuscular blockade. After resolving of neuromuscular blockade sedation should keep the patient pain-free but interactive, calm but lucid and cooperative.76 Over-sedation was associated with prolonged mechanical ventilation and ICU-stay, while light sedation was associated with reduced length of ICU and hospital stay, less post-traumatic stress disorders and improved survival rates.76–78
In order to minimise sedation, the authors use short-acting sedative agents such as propofol or dexmedetomidine (if tolerated from a haemodynamic point of view). A non-benzodiazepine approach in critically ill patients led to a shorter duration of mechanical ventilation, a reduced length of ICU stay79 and a lower mortality80 compared to the use of benzodiazepines. The benefits of dexmedetomidine include the better sleep architecture,81 a lower risk of delirium and a shorter length of stay after cardiac surgery.82 When long-active sedatives such as midazolam must be used due to haemodynamic instability, daily interruption of sedation is mandatory, as this has been shown to reduce days on the ventilator, ICU length of stay and even mortality.78,83
Detect and Treat Delirium
There is extensive evidence that delirium prolongs ICU stay84 and increases morbidity and mortality85–88 after cardiac surgery. Also a longer duration of delirium is associated with worse global cognition after discharge.89 Risk factors for delirium after cardiac surgery include age, preexisting cognitive impairment and cerebrovascular disease, benzodiazepine use and immobilsation.90 A review also pointed out that blood transfusion, mechanical ventilation and even use of intraaortic balloon pump91 are associated with increased risk of delirium. The confusion assessment method for the ICU (CAM-ICU) 92,93 and the intensive care delirium screening checklist (ICDSC) 94 are tools for the diagnosis of delirium in critically ill patients with reported pooled sensitivities and specificities of 80 % and 75 % for the ICDSC and 76 % and 96 % for the CAM-ICU.95 Scarce data exist for the treatment of delirium.88 Cooperative patients with a hypoactive form of delirium and/or hallucinations are treated with haloperidol, preferably orally and with low doses. In the authors’ institution, agitated and noncooperative patients are treated primarily with enteral pipamperon, a mild neuroleptic agent with sedative properties. The drug is usually given in the afternoon and evening (e.g. 4pm, 6pm and 8pm) to treat agitation and to induce sleep. Intravenous dexmedetomidine is added in severely agitated patients.
Rehabilitate Early
Prolonged critical illness results in loss of lean body mass and muscle weakness.96,97 Catecholamines induce myocyte apoptosis, and muscle weakness is potentially related to excessive sympathetic tone.12,98 Physiotherapy in the ICU is safe99 and can be started in cardiac surgery patients considering safety issues which are related to devices, sheaths or sternotomy.100 Early mobilisation and physiotherapy improves functional outcome and probably reduces length of ICU stay.101–103 Even in patients with ECLS, early physiotherapy and mobilisation is possible and safe.104,105
Feeding and Support Metabolism
Enteral Versus Parenteral Nutrition
Early enteral nutrition is recommended to preserve gastro-intestinal integrity and prevent bacterial translocation.106 On the other hand, enteral nutrition bears the risk of vomiting and aspiration, gastrointestinal obstruction and bowel ischaemia. This is particularly true during prolonged shock, when blood is redistributed from the gut to vital organs such as the brain and the heart.
The authors start nutrition with 5 kcal/kg/day after shock resolution (normalised lactate levels, decreasing noradrenaline requirements) and increase stepwise to 25 kcal/kg/day over the next days. In patients without a contraindication for enteral nutrition, an early initiation of parenteral nutritision caused longer ICU stays and higher incidence of ICU aquired infections and higher health care costs.107 Other complications such as poor glycaemic control were described with parenteral nutrition.108,109 Uncertainty exists in patients with contrandications for enteral feeding: a meta-analysis of older studies (between 1981 and 1994) showed an association with higher infection rates in patients with parenteral nutrition,110 whereas a newer study showed a shorter duration of mechanical ventilation (without effect on 60-day mortality) if patients received parenteral nutrition within 24 hours of ICU admission.111
Recently, a study showed benefits of parenteral nutrition was started on day four if enteral intake was <60 % of the targeted calories.112 Therefore, the authors start parenteral nutrition between days four and eight if shock has resolved and enteral calory intake is <60 % of the targeted calories. As with enteral nutrition, parenteral nutrition is gradually established over days, up to 80 % of the target calory intake.
Stress Ulcer Prophylaxis
Following international guidelines, the authors recommend stress ulcer prophylaxis with proton pump inhibitors (e.g. entral or intravenous pantoprazole 40 mg once daily) in patients with risk factor of gastro-intestinal bleeding (coagulopathy or anticoagulation, prolonged mechanical ventilation >48 hours, hypotension, steroid therapy).113 Meta analysis showed significantly less upper gastrointestinal bleeding with prophylaxis (in the absence of any mortality benefit).114–116 It is worth noting that stress ulcer prophylaxis is a grade 2C recommendation in the 2013 surviving sepsis campaign.113 Since there is an increased risk of pneumonia with increased stomach pH in ambulant patients,117 there might be a greater incidence of VAP with use of stress ulcer prophylaxis. Clostridium difficile infections have also be associated with the use of prophylaxis.118 Taking these considerations into account, the use of stress ulcer prophylaxis should be limited to patients at risk of bleeding, until enteral nutrition is fully established.
Blood Glucose Control
Hyperglycaemia is common in patients with shock due to the physiological stress reaction. Excessive glucose plasma levels have been associated with adverse outcome.119,120 However, pharmacological glucose control bears the risk of hypoglycaemia.121 A large, international randomised trial showed that a liberal glucose management (targeting glucose levels <10 mmol/l) resulted in a lower mortality than targeting a glucose level of 4.5–6 mmol/l.122 As numerous arterial blood gas analyses including glucose measurements are performed, the authors have a low rate of accidental hypoglycemic episodes. In their ICU, the authors have agreed on blood glucose targets between 4.5–8.5 mmol/l using continuous insulin infusions.
Use DVT Prophylaxis
As critical ill patients are at risk for deep vein thrombosis (DVT),113,123 prophylaxis is warranted. The decision when to start and how to provide DVT prophylaxis may be difficult as the bleeding risk is high after cardiac surgery. In a prospective multicentre trial independent predictors for major bleeding in patients receiving heparin thrombosis prophylaxis were described, including renal replacement therapy, low platelet count and antiplatelet agents during the past 7 days.124
The authors usually start DVT prophylaxis with a continuous infusion of 10,000 IE/24h of unfractionated heparin 6 hours after surgery. In patients with an indication for therapeutic anticoagulation, the heparin dose is increased by 2,000 to 5,000 IE every 6 hours until the anti-Xa activity is between 0.3 and 0.7 IE/ml.
Infection Control
During the early phase of shock, broad-spectrum intravenous antibiotics are used if sepsis is suspected and after samples for microbiology have been taken. Importantly, antibiotic therapy has to be adapted according to the microbiology results, or even stopped if the patient improves and no pathogen is identified.113
The use of procalcitonin (PCT) as a marker to guide duration of antibiotic therapy reduces length and cost of antibiotic therapy.125 However, PCT levels usually increase after cardiac surgery even without infections.126 PCT should therefore not be used as a sole marker of infection to guide antibiotic therapy in patients after cardiac surgery.
Conclusion
Shock in cardio-surgical patients is a life-threatening disorder. Multiple organ failure can occur despite early diagnosis and prompt therapeutic interventions. The period beyond the first 6 hours of shock onset, also referred to as the silver days, is the focus of the current review. The authors discuss haemodynamic management, ventilation strategies, renal replacement therapy, sedation and nutrition in affected patients. Over the years, allegedly life-saving treatments were shown to have rather harmful effects in critically ill patients, particularly if prolonged use or high dosages are applied. Potentially harmful interventions include haemodynamic optimisation to supranormal targets, excessive fluid administration, over-feeding and oversedation. Hence, treatments of patients during the silver days must focus on reducing harm and avoiding complications related to critical care.4,127 This concept includes avoidance of fluid overload, reduction of excessive catecholamines, safe ventilation and daily interruption of sedation. Although the authors focus on cardio-surgery ICU patients, most of the considerations apply to critical-ill patients in general.