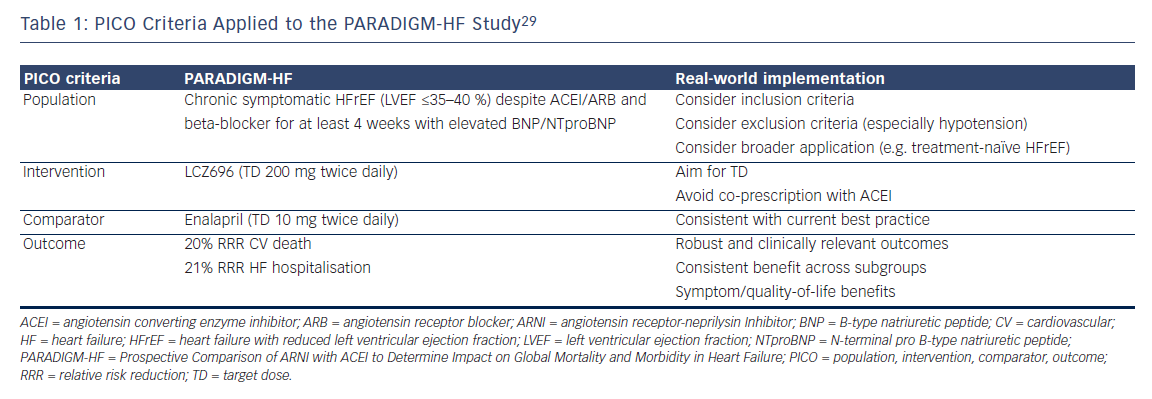
Heart failure (HF) is associated with significant morbidity and mortality and confers a major economic burden.1 Large randomised controlled trials (RCTs) have demonstrated that inhibition of the renin–angiotensin– aldosterone and sympathetic nervous systems improve outcomes in patients with HF and a reduced left ventricular ejection fraction (HFrEF) (see Figure 1),2–9 with clinical guidelines recommending angiotensin-converting enzyme inhibitors (ACEIs), beta-blockers and mineralocorticoid receptor antagonists in all patients with symptomatic HFrEF unless contraindicated.10–12 However, despite the considerable therapeutic gains made in the field of HFrEF, outcomes remain poor especially in patients with persisting left ventricular systolic dysfunction.
Evidence for Angiotensin-converting Enzyme Inhibitors in Heart Failure
The ACEIs was the first class of drug shown to improve survival rates and reduce HF hospitalisation rates in patients with mild, moderate or severely symptomatic HF.2,3 A meta-analysis of RCTs evaluating ACEI in patients with HF reported substantial reductions in total mortality rates (hazard ratio [HR] 0.77; 95 % CI [0.67–0.88]; P<0.001), with consistent benefits across multiple subgroups.4 More recent studies demonstrated that angiotensin receptor blockers (ARBs) also improve outcomes, with clear benefits in patients unable to tolerate ACEIs.13–15 On the basis of these studies, ACEI are given the highest level of evidence in HF clinical guidelines,11,12 with recent registries and real-world studies reporting prescription rates >90 % for ACEI/ARB therapy in eligible patients with HF.16,17
Rationale for Neprilysin Inhibition in Heart Failure
Neprilysin is an enzyme that catalyses the degradation of a number of vasoactive compounds, including natriuretic peptides. Natriuretic peptides have multiple actions that could have a favourable effect on HF disease progression including vasodilation, natriuresis and diuresis;18 thus the promotion of natriuretic peptides through exogenous administration or inhibition of neprilysin are attractive therapeutic options. Intravenous nesiritide, a synthetic B-type natriuretic peptide (BNP), was shown to reduce the rates of dyspnoea and pulmonary capillary wedge pressure compared with placebo in the Vasodilation in the Management of Acute Congestive HF (VMAC) study; however, there was no difference in symptom improvement compared with nitroglycerin.19 Furthermore, nesiritide had no effect on death or rehospitalisation rates in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) study, and is therefore not recommended for routine use.20
The first orally available neprilysin inhibitor, candoxatril, although displaying a dose-dependent increase in atrial natriuretic peptide levels accompanied by natriuresis and haemodynamic benefits in the setting of HF in short-term studies,21,22 was associated with increases in levels of angiotensin II and endothelin, which likely offsets the favourable haemodynamic effects in the absence of renin–angiotensin system inhibition.23,24 Another neprilysin inhibitor, ecadotril, failed to show benefit in a dose-ranging study with a trend towards increased mortality rates.25
The combination ACE–neprilysin inhibitor, omapatrilat, was compared with enalapril in 5,770 patients with HFrEF in the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) study. There was no reduction in the primary endpoint of death or HF hospitalisation, although the secondary endpoint of cardiovascular death or hospitalisation was significantly reduced, as was the primary endpoint in a post-hoc analysis using the Studies of Left Ventricular Dysfunction-Treatment (SOLVD-T) study definition for hospitalisation.26 A higher rate of angioedema, especially in the setting of hypertension (including rare reports of severe cases) led to withdrawal of the drug.27 The increased rate of angioedema was theoretically attributed to inhibition of ACE, neprilysin and aminopeptidase-P, which are all involved in bradykinin breakdown, given that increased levels of plasma bradykinin has been documented during acute angioedema episodes.28
LCZ696 is a first in the angiotensin receptor–neprilysin inhibitor (ARNI) class that combines the effects of angiotensin receptor blockade with valsartan and neprilysin inhibition with sacubitril. This was designed to have a reduced risk of angioedema compared with omapatrilat, because it does not inhibit ACE or aminopeptidase-P. The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study was stopped early by the data and safety monitoring committee because of overwhelming benefit of LCZ696 compared with enalapril in 8,442 patients with HFrEF.29 There was a highly significant reduction in the primary endpoint of cardiovascular death or HF hospitalisation (HR 0.80; 95 % CI [0.71–0.87]; P<0.001), driven by significant reductions in both cardiovascular death and HF hospitalisation rates. The secondary endpoint, all-cause mortality, was also reduced, accompanied by beneficial effects on quality of life. LCZ696 was well tolerated with fewer patients randomised to receive LCZ696 stopping treatment because of an adverse event compared with the enalapril group.29
Applying the Evidence for Angiotensin Receptor–neprilysin Inhibitors to Clinical Practice
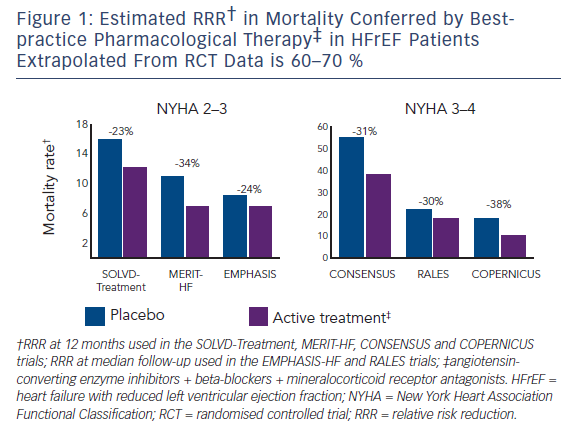
When determining whether the results of a clinical trial can be applied to clinical practice, one should consider the population studied, the intervention, the comparator and the outcome measures (referred to as the PICO approach – see Table 1). In the PARADIGM-HF study patients with chronic HFrEF with a left ventricular ejection fraction (LVEF) ≤40 % (later lowered to ≤35 %) were evaluated. Patients had to be symptomatic with an elevated BNP level (or N-terminal proBNP [NTproBNP]) ≥150 (≥600) pg/ml, or ≥100 (≥400) pg/ml if they had been hospitalised for HFrEF within the previous 12 months. They were required to be on a stable dose of an ACEI or an ARB (equivalent to ≥10 mg enalapril daily), and a beta-blocker for at least 4 weeks. Mineralocorticoid receptor antagonists were encouraged.29 The trial design included a single-blind, run-in phase to ensure that patients could tolerate the recommended target doses for both treatment arms. The patients were generally well treated with high levels of evidencebased pharmacological therapies including beta-blockers (93 %) and mineralocorticoid receptor antagonists (60 %).
The intervention evaluated in the PARADIGM-HF study was dual blockade of the renin–angiotensin system and neprilysin.29 This study was not designed to test whether the benefit of LCZ696 was dose related, nor whether the relative effect on these neurohormonal systems varied at different doses. In other words, we cannot assume that superiority over an ACEI (presumably related to the additional effect of neprilysin inhibition) will be maintained at low doses of LCZ696. It is therefore important that when clinicians prescribe LCZ696, they aim for the target dose tested in the PARADIGM-HF study.
An ACEI was chosen as the comparator in the PARADIGM-HF study, given its robust evidence for safety and clinical effectiveness in HFrEF, and that ACEIs are recommended as first-line therapy in all major HF clinical guidelines.11,12 Enalapril was specifically chosen because it has been shown to reduce mortality rates in patients with chronic HFrEF; and the target dose of 10 mg twice daily was the same as that in the SOLVD-T study.3,29 Indeed, the mean daily dose achieved in the PARADIGM-HF study was 18.9 mg, which was higher compared with that in previous HF studies.2,3
The outcome measures chosen in PARADIGM-HF study were robust and clinically relevant, including beneficial effects on symptoms, quality of life, rates of hospitalisation and other health resource utilisation, and mortality rates.29,30 Furthermore, there were no subgroups where the point estimate HR was >1.0. The only pre-specified subgroup with a nominally significant interaction for the primary endpoint (unadjusted for multiple comparisons) was New York Heart Association class; however, there was no significant interaction effect for cardiovascular death. The benefits were observed on top of background therapy with 93 % of patients receiving beta-blockers at the time of randomisation. Although only 55 % of patients were receiving a mineralocorticoid receptor antagonist at the time of randomisation, significant reductions in the primary endpoint and cardiovascular death were observed in patients with or without prior mineralocorticoid receptor antagonist therapy.29 Furthermore, significant reductions in sudden death rates were observed in both patients with and without an implantable defibrillator device.31 Only 7 % of patients had a cardiac resynchronisation therapy device at the time of randomisation; however, the benefits of LCZ696 and cardiac resynchronisation therapy should be maintained in patients who meet the inclusion criteria for these treatments, including a persistent moderate to severe reduction in LVEF.
Limitations of the Evidence for Angiotensin Receptor–neprilysin Inhibitors in Heart Failure
The PARADIGM-HF study is the only one supporting the use of ARNI over ACEI in patients with HFrEF. It is nonetheless a large study that was primarily powered to detect a difference in cardiovascular mortality rates. Indeed, the P value achieved for the primary endpoint was equivalent to at least four trials with a P value <0.05.32 On this basis, it would appear unethical to conduct a similar study to confirm the PARADIGM-HF findings.
The most appropriate population to receive ARNI in clinical practice would match those who were studied in the PARADIGM-HF study, namely patients with symptomatic HFrEF despite appropriate doses of ACEI (or ARB) and beta-blockers. Although a clinical trial investigator may argue that the inclusion criteria for a clinical trial should be applied in clinical practice, one should also consider whether this allows clinicians to identify those patients most likely to benefit. For example, the patients enrolled in the PARADIGM-HF study had elevated BNP/NTproBNP levels; however, there was no significant interaction effect for the primary endpoint according to baseline BNP/NTproBNP levels. Therefore, this would not meet the diagnostic test requirements for a co-dependent technology to determine treatment eligibility.
Closer attention should be applied to the exclusion criteria in the PARADIGM-HF study.29 These included hypotension, estimated glomerular filtration rate below 30 ml/min/1.73 m2 of body surface area, hyperkalaemia, and a history of angioedema or unacceptable side effects to ACEI or ARB. Given that fewer patients in the LCZ696 group experienced a serum creatinine level of ≥221 μmol/l or a serum potassium level of >6.0 mmol/l, it would appear that the same restrictions for renal impairment and hyperkalaemia should be applied to ARNI and ACEI. However, hypotension was more common with LCZ696, although this did not lead to more treatment withdrawals. Nonetheless, hypotension may limit uptitration of other disease-modifying therapies including betablockers, and the relative efficacy of low doses of LCZ696 compared with ACEI is unknown. Therefore, it would seem appropriate to avoid LCZ696 and favour ACEI or ARB therapy (at least initially) in patients with symptomatic hypotension or systolic blood pressure <95–100 mmHg.
As with most studies that have demonstrated the safety and clinical efficacy of treatments in HF, the patients in the PARADIGM-HF study were on average a decade younger with fewer co-morbidities compared with those enrolled in clinical registries.33 Although there was no significant interaction between treatment efficacy and age, systolic blood pressure, or the presence or absence of diabetes mellitus or chronic kidney disease for the patients enrolled in PARADIGM-HF study,29 clinicians will need to balance the safety and efficacy of LCZ696 in the broader HFrEF population.
Patients with a new diagnosis of HFrEF were not evaluated in the PARADIGM-HF study.29 Such treatment-naïve patients would be more likely to experience side effects, and the relative efficacy of ARNI may be reduced, given that a proportion of HFrEF patients clinically improve with substantial reverse remodelling on current best-practice therapy. One approach could be to start standard therapy (including an ACEI, beta-blocker and mineralocorticoid receptor antagonist) in all patients with a new diagnosis of HFrEF, and to switch the ACEI to LCZ696 in those patients with persistent HFrEF after ≥4 weeks. This may involve repeating the assessment of LVEF, which is currently common practice after 3–6 months in patients with a new diagnosis of HFrEF. However, such an approach would further complicate the medication uptitration process and needs to be tempered with the recently reported early benefits experienced in the LCZ696 group in the PARADIGM-HF study, including a significantly lower rate of HF hospitalisation by 30 days.30 An alternative and reasonable approach would be to prescribe an ARNI in all patients with HFrEF (including those with a new diagnosis) provided there are no contraindications such as previous angioedema or significant hypotension; however, this will be guided by local regulatory and reimbursement processes.
This review has not considered cost effectiveness or total healthcare costs, which will be of interest to payers and jurisdictions that provide reimbursement for pharmaceuticals. Finally, implementing ARNI into clinical practice will need to be accompanied by broad education of multiple healthcare professionals including primary care physicians, pharmacists, HF nurses, cardiologists and geriatricians. This will be particularly important to ensure appropriate prescribing to avoid leakage to patients unlikely to benefit and avoid inadvertent co-prescription of ARNI and ACEI, given that ACEI have been recommended as first-line HFrEF treatment for over two decades.
Conclusion
The superiority of ARNI compared with ACEI in chronic HFrEF has been conclusively demonstrated in the PARADIGM-HF study. Although clinical practice will be guided by local regulatory approvals, there is little reason to think that this would not be applicable to the broader HFrEF population, including treatment-naïve patients. However, this should not be at the expense of other disease-modifying therapies, such as betablockers. Finally, given the well-established role of ACEI for over two decades, implementation of ARNI will need to be accompanied by broad education of healthcare professionals involved in HF management.