In 2008, the European Medicines Agency and US Food and Drug Administration (FDA) issued industry guidance stating that all future novel glucose-lowering agent trials must undergo routine cardiovascular risk evaluation either before approval or as a post-marketing commitment.1 This mandated that all cardiovascular endpoint committees prospectively adjudicate all major adverse cardiovascular events, including cardiovascular death, non-fatal MI and stroke, occurring across Phase II and III diabetes trials. However, the statement did not specifically mention heart failure (HF) as an endpoint.
HF is the second most common cardiovascular presentation of diabetes after peripheral arterial disease.2 In people with diabetes, mortality from HF constitutes a large proportion of overall mortality. Moreover, approximately 40% of hospitalised HF patients have concomitant diabetes, a trend that is expected to increase even further.3–5 Both diabetes and HF are closely linked, with one causing a worse prognosis in the other. The majority of anti-hyperglycaemic agents primarily reduce the risk of ischaemic microvascular events without targeting the mechanisms involved for diabetes cardiomyopathy and HF.
Some glucose-lowering agents, such as thiazolidinediones and saxigliptin, have been linked to increased risk of incident HF and HF hospitalisations.6,7 Other drugs, such as liraglutide, have shown overall cardiovascular benefit without specifically improving outcomes in established HF patients.8 However, unlike other anti-hyperglycaemics, sodium–glucose cotransporter-2 (SGLT2) inhibitors have emerged as a novel class of glucose-lowering agents that have consistently reduced HF hospitalisations.9–15 This class of medication works by selectively inhibiting SGLT2, thus causing decreased renal absorption of glucose. Multiple studies have shown that SGLT2 inhibitors are associated with weight loss and blood pressure reduction in addition to glycaemic control.16 In this article, we discuss the current evidence and highlight the future direction for SGLT2 inhibitors in HF prevention.
Evidence from Randomised Clinical Trial and Observational Data
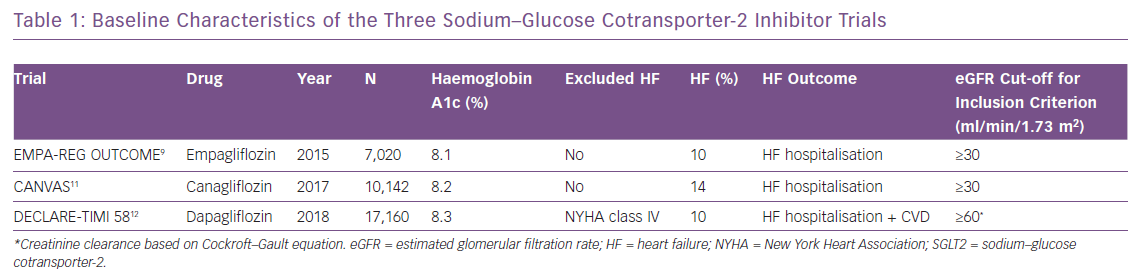
There are three completed cardiovascular safety trials for SGLT2 inhibitors: EMPAgliflozin cardiovascular outcome event trial in type 2 diabetes – Removing Excess Glucose (EMPA-REG OUTCOME), CANagliflozin cardioVascular Assessment Study (CANVAS) and Dapagliflozin Effect on CardiovascuLAR Events – Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58).9,11,12
In EMPA-REG OUTCOME, 7,020 people with diabetes and established cardiovascular disease were randomised to empagliflozin or placebo.9 The primary endpoint of the trial – major adverse cardiovascular events – was significantly reduced (HR 0.86; 95% CI [0.74–0.99]; p=0.04) in the empagliflozin arm. HF hospitalisation was an undefined secondary outcome. Compared with placebo, empagliflozin significantly reduced the risk of HF hospitalisations (4.1% versus 2.7%; HR 0.65; 95% CI [0.50–0.85]) and composite outcome of HF hospitalisations and cardiovascular death (5.7% versus 8.5%; HR 0.66; 95% CI [0.55–0.79]; p<0.001). Consistent benefit in HF hospitalisation was observed in all subgroups including age, race, estimated glomerular filtration rate (eGFR) and baseline medications for HF. Empagliflozin was also beneficial across a spectrum of HF patients.17 The results of these trials led to empagliflozin being approved by the FDA in 2016 for the prevention of major adverse cardiovascular events in people with diabetes.18 However, no specific recommendation was given for HF prevention, as HF was not one of the primary endpoints.
In the CANVAS trial,10,142 people with diabetes and high cardiovascular risk were randomised to canagliflozin or placebo.11 The primary endpoint was major adverse cardiovascular events, which was significantly reduced in the canagliflozin group (HR 0.86; 95% CI [0.75–0.97]; p<0.001 for non-inferiority; p=0.02 for superiority). Canagliflozin was also associated with a significant reduction in the risk of HF hospitalisation (5.5 versus 8.7 per 1,000 patient-years; HR 0.67; 95% CI [0.52–0.87]). Subgroup analyses showed that patients with baseline HF derived a greater benefit in terms of cardiovascular death and HF hospitalisations.
In the recent DECLARE-TIMI 58 trial, which evaluated 17,160 patients, dapagliflozin also significantly reduced the risk of HF hospitalisation (6.2 versus 8.5 per 1,000 patient years; HR 0.73; 95% CI [0.61–0.88]).12 Approximately 4% of patients had HF with reduced ejection fraction (HFrEF) at baseline. Dapagliflozin was shown to reduce the composite endpoint of cardiovascular death/HF hospitalisation more in patients with HFrEF (HR 0.62; 95% CI [0.45–0.86]) compared to those without HFrEF (HR 0.88; 95% CI [0.76–1.02]; p-interaction 0.046). The borderline non-significant results in the non-HFrEF patients were mainly driven by cardiovascular death as in the subgroup analysis; dapagliflozin decreased HF hospitalisation both in patients with HFrEF (HR 0.64; 95% CI [0.43–0.95]) and without HFrEF (HR 0.76; 95% CI [0.62–0.92]). However, the statistically significant reduction in cardiovascular death was observed only in the HFrEF group (HR 0.55; 95% CI [0.34–0.90]).
The DECLARE-TIMI 58 trial was unique compared with the previous two trials because it enrolled more patients without known atherosclerotic cardiovascular disease (n=10,186) and was the first trial to include HF hospitalisation as the co-primary endpoint. The baseline HF rate in all three trials was <15%. Table 1 shows baseline characteristics of the three SGLT2 inhibitor trials, and Table 2 shows detailed HF outcomes.
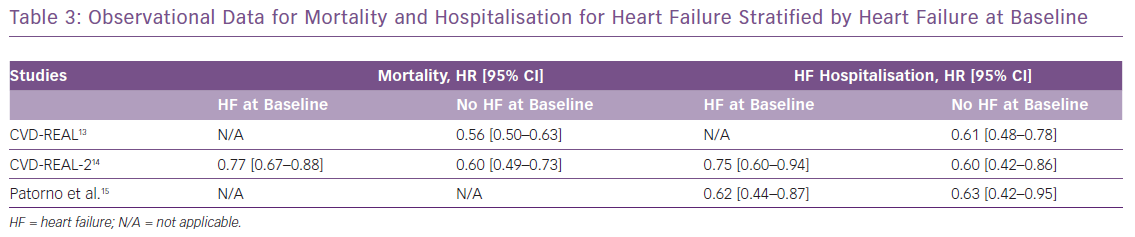
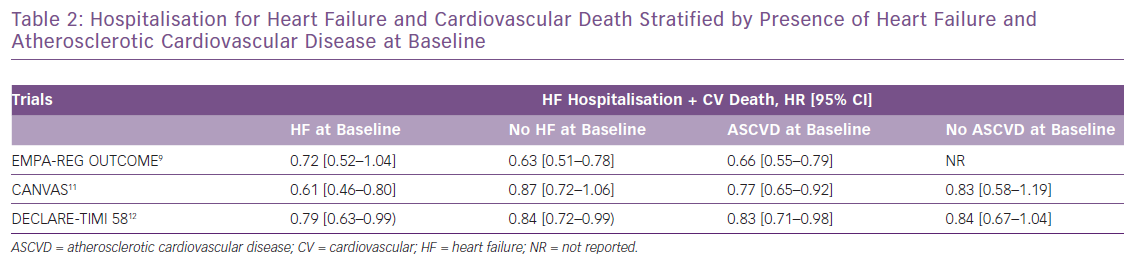
These clinical trial data are further supported by the real-world evidence from observational studies.13–15 The Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors study (CVD-REAL), comprising more than 300,000 newly diagnosed diabetes patients, compared those who were initiated on SGLT2 inhibitors with those receiving any other glucose-lowering therapy. SGLT2 inhibitors led to an almost 40% relative reduction in HF hospitalisation compared with other therapies.13 A consistent benefit was seen in mortality and HF hospitalisation across the spectrum of patients, including those with or without HF at baseline. Moreover, a network meta-analysis suggested that SGLT2 inhibitors have 99.6% probability of being the most effective anti-hyperglycaemic agent for reducing the risk of HF hospitalisation.19 Table 3 shows the main HF outcomes from the real-world observational data of SGLT2 inhibitors.
Mechanism for Benefit
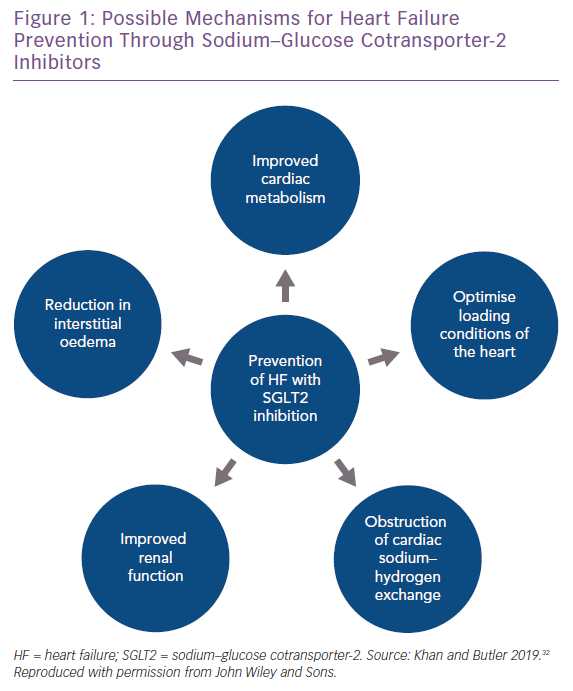
The cardioprotective effects offered by SGLT2 inhibitors cannot be solely attributed to glycaemic control. Several mechanisms for the beneficial effect of SGLT2 inhibitors in regards to HF have been proposed and are highlighted in Figure 1.
First, SGLT2 inhibitors have a direct effect on cardiac metabolism by increasing hepatic neogenesis of ketone bodies, which serve as the alternate fuel for a hypertrophied and failing heart.20 Second, SGLT2 inhibitors are believed to inhibit myocardial and renal sodium–hydrogen exchanger 3, leading to modification of intracellular calcium and thus prevention of HF-associated remodelling.21 Third, improvement in renal function and interstitial volume regulation by SGLT2 inhibitors may also contribute to improvement in HF risk.22 Finally, SGLT2 inhibitors cause osmotic diuresis through glycosuria and natriuresis, which may help in optimising loading conditions of the myocardium. SGLT2 inhibitors have also been shown to significantly reduce blood pressure and biomarkers of arterial stiffness, which can lead to better oxygen consumption of the heart.20–22 These mechanisms – aside from glycaemic control – also suggest a role for the use of SGLT2 inhibitors solely for HF prevention, regardless of diabetes status.
Effect on Subgroups
In the early SGLT2 inhibitor trials, the benefit for major adverse cardiovascular events seemed to be higher in patients with established atherosclerotic cardiovascular disease, although formal heterogeneity was not shown. This led to the American and European guidelines recommending the use of SGLT2 inhibitors in people with diabetes and atherosclerotic cardiovascular disease.23,24 However, a recent meta-analysis including data from EMPA-REG OUTCOME, the CANVAS Program and DECLARE-TIMI 58 showed that SGLT2 inhibitors reduce HF hospitalisations regardless of the presence of HF or atherosclerotic cardiovascular disease at baseline.25 There was an approximately 30% relative risk reduction for HF hospitalisation in both the subgroups. Interestingly, the benefit for major adverse cardiovascular events was only limited to patients with baseline atherosclerotic cardiovascular disease. Thus, data suggest that SGLT2 inhibitors are especially beneficial for HF over a broad spectrum of patients with diabetes. Studies have also demonstrated that the beneficial effect of SGLT2 inhibitors on HF hospitalisation is similar in males and females.26
Reno-protective Effects of SGLT2 Inhibitors
A possible explanation for SGLT2 inhibitors reducing HF hospitalisations, regardless of the presence of HF or atherosclerotic cardiovascular disease at baseline, is the reno-protective effects of SGLT2 inhibitors coupled with natriuresis. There are four studies that have assessed the effect of SGLT2 inhibitors on renal outcomes: the Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE), EMPA-REG OUTCOME, the CANVAS Program and DECLARE-TIMI 58. EMPA-REG OUTCOME, the CANVAS Program and DECLARE-TIMI 58 were primarily designed as cardiovascular outcome trials with a range of pre-specified exploratory and post hoc renal outcomes.
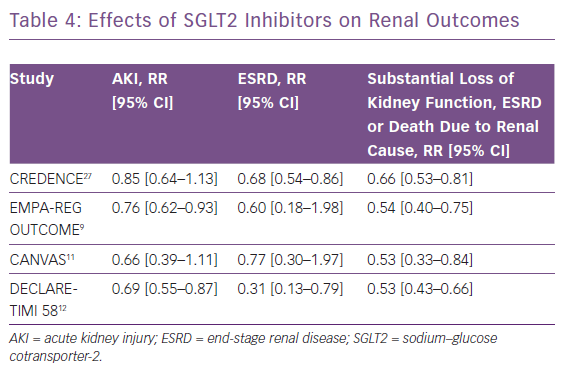
The CREDENCE trial, published in 2019, was the first study to specifically determine the effect of SGLT2 inhibitors on renal outcomes in patients with already-established diabetic kidney disease.27 The primary composite outcome of the CREDENCE trial was doubling of serum creatinine, end-stage renal disease or mortality due to cardiovascular or renal cause. The relative risk of the primary outcome was 30% lower in the SGLT2 inhibitor group compared with the placebo group (event rates of 43.2 and 61.2 per 1,000 patient-years, respectively; RR 0.70; 95% CI [0.59–0.82]; p=0.00001). On pooling results from all the four studies, SGLT2 inhibitors were shown to decrease the risk of dialysis, transplantation or mortality due to renal disease by approximately one-third.28 SGLT2 inhibitors were also shown to decrease the risk of acute kidney injury by 25%. Results from each of the individual studies on various renal endpoints are shown in Table 4. Moreover, significant evidence of benefit was apparent for all eGFR subgroups,, including for patients with a baseline eGFR <45 ml/min/1.73 m2.28 An eGFR rate of ≥30 ml/min/1.73 m2 was an inclusion criterion for all four studies apart from DECLARE-TIMI 58, in which a creatinine clearance of ≥60 ml/min was used. Similar to the HF effect, the reno-protective effect was robust in both patients with and without atherosclerotic cardiovascular disease. It is known that patients with lower eGFR are at higher risk for HF hospitalisation, and thus SGLT2 inhibitors conferring reno-protection and natriuresis could be the main contributing mechanism for HF prevention.29,30
Current Position and Future Direction
In the current guidelines published by the American Diabetes Association and European Association for the Study of Diabetes, metformin remains the first-line treatment for people with diabetes, with SGLT2 inhibitors as the second-line therapy.31 Given the absence of any mortality or cardiovascular benefit with metformin, future studies should investigate the role of SGLT2 inhibitors as a first-line therapy.32 Moreover, the role of SGLT2 inhibitors in stage D HF patients remains unknown. Considering that stage D HF patients often do not tolerate HF therapy, SGLT2 inhibitors might be an attractive alternative.33
Although SGLT2 inhibitors have shown substantial improvement in HF outcomes, data collected need to be further expanded to include ejection fraction and New York Heart Association (NYHA) class, allowing for more specific subgroup analyses. Moreover, endpoints apart from HF hospitalisation should be considered, such as emergency department visits and urgent office visits. A recent systematic review has highlighted significant gaps regarding HF data capture in novel glucose-lowering therapy trials.34 Apart from EMPA-REG OUTCOME, none of the SGLT2 inhibitor trials provided details of how HF was defined at baseline. EMPA-REG OUTCOME defined HF using a query for cardiac failure through the Medical Dictionary for Regulatory Activities. None of the trials reported brain natriuretic peptide data, ejection fraction or degree of optimisation in patients who had baseline HF. Moreover, no trial commented on outcome data once the patient had incident HF.
There are three on-going trials that will further evaluate the role of SGLT2 inhibitors for HF treatment, irrespective of diabetes status – EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR-Reduced; NCT03057977), EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Preserved Ejection Fraction (EMPEROR-Preserved; NCT03057951) and the Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure [DAPA-HF]; NCT03036124).
EMPEROR-Reduced and EMPEROR-Preserved will randomise patients to empagliflozin or placebo with cardiovascular death and HF-related hospitalisation as the primary endpoint. Similarly, DAPA-HF will randomise patients to dapagliflozin or placebo with cardiovascular death and HF-related hospitalisation as the primary endpoint. Inclusion criteria include established HFrEF (NYHA class II–IV) and ejection fraction ≤40%. There is also an on-going trial evaluating ertugliflozin in diabetes patients with vascular disease: Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease ([VERTIS CV]; NCT01986881). VERTIS CV is expected to enrol 8,000 patients with a primary outcome of time to first occurrence of major adverse cardiovascular event (cardiovascular death, non-fatal MI or stroke). Secondary endpoints include HF-related hospitalisation.
If these trials show improvement in HF outcomes with SGLT2 inhibitors, it would represent a paradigm shift in management of HF. While we await results from these trials, it is important to acknowledge that we already have strong evidence that SGLT2 inhibitors provide benefit for primary HF prevention in people with diabetes. Given the robust data and dire public health consequences of concomitant diabetes and HF, clinicians should consider initiating SGLT2 inhibitors for HF prevention at least in diabetes patients, regardless of their HbA1c and atherosclerotic disease status.