Advances in the early detection and treatment of cancer have improved overall survival in cancer patients. Nevertheless, cardiovascular diseases appear as the major cause of morbidity and mortality among cancer survivors.1 Left ventricular (LV) dysfunction and/or heart failure are the most common cardiovascular complications after administration of chemotherapies. The term ‘cardiotoxicity’ is generally used to refer to LV dysfunction. The two main anticancer agents responsible for LV dysfunction are anthracyclines and targeted therapies (tyrosine kinase inhibitor, anti-human epidermal growth factor receptor 2, anti-vascular endothelial growth factor, proteasome inhibitors). Recently, immune fulminant myocarditis was reported with the use of checkpoint immune inhibitors (anti-programmed cell death protein 1, anti-programmed cell death ligand 1, anti-cytotoxic T lymphocyte-associated protein 4), suggesting new cardiotoxicity pathways.2 LV dysfunction remains asymptomatic for a long time, but once symptomatic, the prognosis is one of the poorest in the heart failure population.3 The challenge is then to detect myocardial toxicity before symptomatic heart failure.
The aim of this review was to define subclinical LV dysfunction during chemotherapy, to identify the best early strategy for cardiodetection, and to summarise the studies’ results about a cardioprotective treatment for cancer patients with subclinical LV dysfunction.
Definition of Left Ventricular Dysfunction Induced by Cardiotoxic Chemotherapies
LV dysfunction induced by cardiotoxic chemotherapies is defined by a decrease in left ventricular ejection fraction (LVEF) of >10 percentage points to a value <53%.4 To detect early myocardial damage before a change in LVEF, an increase in biomarkers levels (mainly troponin I; TnI) and a decrease of >15% of the global longitudinal strain are useful tools.
According to the European Society of Cardiology, LVEF assessment can be performed by echocardiography (attempting to favour 3D LVEF), cardiac nuclear imaging and cardiac MRI.2,5
Detecting Subclinical Left Ventricular Dysfunction Before LVEF Decrease
Identifying High-risk Patients
The paediatric population (aged <18 years) or older patients (aged >65 years) have an increased risk of cardiotoxicity. Moreover, cardiovascular risk factors (arterial hypertension, diabetes, hypercholesterolaemia or family history of cardiovascular disease), current myocardial disease, previous cardiotoxic cancer therapies or lifestyle risk factors (smoking, alcohol, sedentary habits, obesity) are associated with LV dysfunction related to cancer therapy. A careful baseline evaluation and cardiac monitoring during and after treatment of these patients is recommended.5
Monitoring Using Cardiac Biomarkers
Troponins (Troponin I and Troponin T)
Cardinale et al. measured TnI in 703 breast cancer patients before chemotherapy, 3 days after and 1 month after chemotherapy, and showed that elevated TnI was able to predict cardiovascular events (cardiovascular mortality, pulmonary oedema, LVEF <25%, arrhythmias).6 Patients with an early or persistent increase of TnI ≥0.08 ng/ml had a higher incidence of cardiac events (37% and 84% respectively; p<0.001).
In a cohort of 204 patients, including 133 with breast cancer, the same authors studied TnI changes in those receiving high-dose chemotherapy, measured before the start of the chemotherapy and at 12, 24, 36 and 72 hours after. In 53% of cases, an increase in TnI occurred within 72 hours after chemotherapy. At the end of chemotherapy, a decrease of LVEF was observed in the TnI+ and TnI− groups, but LVEF decline was significantly lower in the TnI− group. At 10 months, LVEF was still impaired in the TnI+ group, whereas it remained at the baseline level in the TnI− group. TnI levels were negatively correlated with the LVEF changes (r = –0.87, p<0.0001).7
In 2010, Cardinale et al. measured TnI in 251 breast cancer patients treated with targeted anti-human epidermal growth factor receptor 2 therapy.8 They observed a TnI increase in 36 patients (14%), most of the time after the first cycle of trastuzumab. In patients developing cardiotoxicity (defined by LVEF decrease >10 units and <50%), plasmatic TnI was 32 pg/ml versus 17 pg/ml in patients without cardiotoxicity (p<0.05). A total of 62% of patients with TnI increase developed LV dysfunction (versus 5% of patients without TnI increase; p<0.001). In addition, those patients were less likely to recover from LV dysfunction and had more cardiovascular events.
More recently, a study of patients receiving trastuzumab and lapatinib (tyrosine kinase inhibitor) after anthracycline chemotherapy also showed that an increase in TnI preceded the maximal LVEF decrease.9
In metastatic kidney cancer patients treated with sunitinib (tyrosine kinase inhibitor), a prospective study revealed an increase of troponin T in 10% of cases. Almost all the patients (8/9) with troponin increase had a LV dysfunction.10 Ederhy et al. reported that in metastatic solid tumours of patients treated with anti-vascular endothelial growth factor and tyrosine kinase inhibitors, TnI elevation occurred in 11%, but this was not due to myocarditis or acute coronary syndrome.11 TnI elevation was not associated with a decrease in LVEF at the time of the patients’ inclusion. The lack of prospective follow-up prevented the authors from knowing if these patients with a TnI increase had a LVEF decline.
Several studies failed to prove the troponin value to detect cardiotoxicity.12,13 These different results may be explained by different troponin assay (T or I, hypersensitive or not), different definitions of cardiotoxicity, and different populations and treatments (type, doses).
Natriuretic Peptides
Lenihan et al. performed a study of 109 patients receiving anthracyclines, in which 10% had a cardiac event (LV dysfunction, arrhythmias, sudden death, sudden cardiac arrest).14 All of these patients had at least one brain natriuretic peptide (BNP) level >100 μg/ml. The biomarkers were assayed before each cycle of chemotherapy and 24 hours later.
In a study conducted by Pichon et al. of 79 women treated for breast cancer with anthracyclines, a BNP level >51.3 ng/l predicted cardiotoxicity (defined by a LVEF decrease in ventriculography) with a sensitivity of 83% and specificity of 90%.15
Sandri et al. reported that a persistently elevated N-terminal pro BNP (NT-proBNP) after anthracycline administration predicts LV dysfunction.16 In breast cancer patients treated with anthracyclines and trastuzumab, De Iulius et al. found elevated NT-proBNP levels at each cycle of chemotherapy, whereas LVEF was not modified. This correlated with the 1-year mortality.17
In a study of 159 metastatic renal cell carcinomas in patients treated with tyrosine kinase inhibitors, 43 developed cardiotoxicity (defined by elevated NT-proBNP and LVEF drop). Of note, 12 of 38 patients with increased NT-proBNP developed a LV dysfunction.18
In a prospective study of metastatic renal cell carcinoma treated with sunitinib, a LVEF decline of 1.9% was found after the first cycle of chemotherapy, but was not associated with any changes in BNP level.19
It should be noted, however, that many studies have shown no correlation between an increase of NT-proBNP or BNP and cardiac dysfunction.20,21 Indeed, Daugaart et al. showed no correlation between baseline BNP or BNP changes with LVEF after chemotherapy.22
The main reasons for the negative results are the lack of a standardised range. Elderly cancer patients often have worse renal function and also higher natriuretic peptide levels.
Other Proposed Biomarkers
Myeloperoxidase is a marker of oxidative stress, one of the key elements in the pathophysiology of anthracycline cardiotoxicity. In a cohort of 78 patients with breast cancer undergoing doxorubicin and trastuzumab therapy, an increase of plasmatic myeloperoxidase was associated with a greater risk of cardiotoxicity (defined by an asymptomatic reduction of LVEF of >10% to <55%; HR 1.34, p=0.04).23 The subgroup of patients with myeloperoxidase and troponin increases developed higher cardiotoxicity.
MicroRNAs (miRNAs) are small, non-coding RNA molecules that play an important role in the regulation of gene expression. They can be associated with cardiovascular diseases. In 24 children treated with anthracyclines compared with nine children treated with non-cardiotoxic chemotherapies, plasma miRs-29b and -499 levels were upregulated 6–24 hours after anthracycline administration. The authors found a correlation between miRs expression, anthracycline doses and troponin T increase.24 In a breast cancer population treated with doxorubucin, the circulating level of miR-1 was associated with LVEF decrease and was better than TnI to discriminate patients who develop cardiotoxicity.25
Some biomarkers of inflammation have been studied. Mercuro et al. showed an interesting correlation between interleukin-6 increase and systolic function decrease in a epirubicin-treated population.26 Somehow, Ky et al. did not find any correlation between C-reactive protein rise and cardiotoxicity in a breast cancer population treated with doxorubicin and trastuzumab.23
Beer et al. also reported through proteomic profiling that high baseline immunoglobulin E levels were associated with a lower risk of cardiotoxicity in doxorubicin- and trastuzumab-treated cancer patients.27
Monitoring Using Cardiac Imaging
For years, LVEF was the only parameter monitoring method that detected cardiotoxicity, and multigated acquisition was the most common method used by oncologists. In the past 10 years, 2D and 3D echocardiography has become the standard for myocardial function assessment. Diastolic dysfunction or Doppler imaging have been promising parameters to detect subclinical cardiotoxicity, but current research is focusing on myocardial deformation analysis.4
Deformation Imaging by 2D Echocardiography
Global longitudinal strain (GLS) is a strong predictor of cardiovascular morbidity and mortality in several cardiac diseases, and seems to be a consistent marker of cardiotoxicity.
Negishi et al. showed that GLS was significantly decreased in 24 patients developing cardiotoxicity (defined as a >10% decline from baseline LVEF). They also observed that an 11% decrease of GLS predicts cardiotoxicity (sensitivity 65% and specificity 94%).28 At 12-month follow-up, longitudinal strain was still associated with LVEF decline.29
Recently, in a population of patients with haematological diseases (lymphoma, leukaemia) treated with anthracyclines, GLS <–17.45% after 150 mg/m² of doxorubicin had a sensitivity of 67% and a specificity of 97% for the detection of cardiotoxicity at 1 year (defined as a decrease of >10% of the LVEF with a LVEF <53%).30
The expert consensus of the American Society of Echocardiography and the European Association of Cardiovascular Imaging considered a 15% reduction of GLS as a significant change to detect cardiotoxicity.4
An Integrated Approach
In a small study of 44 patients treated with anthracycline and trastuzumab, Sawaya et al. showed that a 10% decrease of GLS combined with an increase of TnI from baseline to 3 months had an 83% positive predictive value and an 89% negative predictive value to detect cardiotoxicity (as defined as a symptomatic decrease >5% of LVEF with LVEF <55% or an asymptomatic decrease >10% with LVEF <55%).41
The same team showed, in a breast cancer population treated with anthracycline and trastuzumab, that a GLS >–19% and a TnI >30 pg/ml have less sensitivity, but a 93% specificity to detect cardiotoxicity.31
Integrated biomarkers and cardiac imaging appears as a promising approach to precisely detect and predict cardiotoxicity.
3D Echocardiography
3D LVEF was shown to have the lowest temporal variability.32 A recent study of breast cancer patients has suggested that nadir LVEF values were identified by 3D echocardiography earlier than 2D echocardiography, suggesting that 3D measured LVEF might be a useful method to identify early cardiac injury.33 3D LVEF and myocardial strain were associated with concurrent and subsequent changes in 2D LVEF, and concurrent change in diastolic function (E/e’). When adjusted for the respective 2D parameters, post-anthracycline 3D LVEF and global circumferential strain predicted subsequent 2D LVEF.
Cardiac Magnetic Resonance
Cardiac magnetic resonance (CMR) is particularly interesting in the cancer population, because of its spatial and temporal resolution, its reproducibility and accuracy for LVEF assessment. Recent evidence suggests that LV global circumferential strain and GLS measured with feature-tracking CMR may also identify early LV dysfunction.34 CMR also helps us to better understand that the decrease in LVEF and strain in cancer patients receiving chemotherapy is partly explained by the decrease in LV end diastolic volume due to a decrease in preload (vomiting, diarrhoea, sepsis leading to dehydration); therefore, LV end diastolic volume and LV end systolic volume should always be taken into account.35 Increased LV afterload represents a condition that may promote an increase in LV end systolic volume related to a factor extrinsic to the LV myocardium; for example, increased vascular resistance resulting from administration of tyrosine kinase inhibitors or endothelium receptors antagonists.36
In cancer patients receiving trastuzumab, a decrease of global circumferential strain and GLS with an increase of LV end diastolic volume seems to predict LV dysfunction.37
CMR may facilitate our understanding for cardiotoxicity pathogenesis. Myocardial tissue changes, such as intracellular and interstitial oedema, and fibrosis, may precede the alterations in LV volumes, reduction in LVEF, or changes in myocardial strain. and may represent early markers of myocardial injury. Also, there is accumulating evidence of the presence of diffuse interstitial fibrosis (assessed by increased T1 mapping and extracellular volume fraction in anthracycline-induced cardiomyopathy), independent of cardiovascular comorbidities and associated with impaired diastolic function.38 There are also many aetiologies of myocellular dysfunction that lead to LV dysfunction in patients receiving cardiotoxic chemotherapies that CMR can diagnose: myocarditis, stress-induced cardiomyopathy, myocellular injury and interstitial fibrosis.39
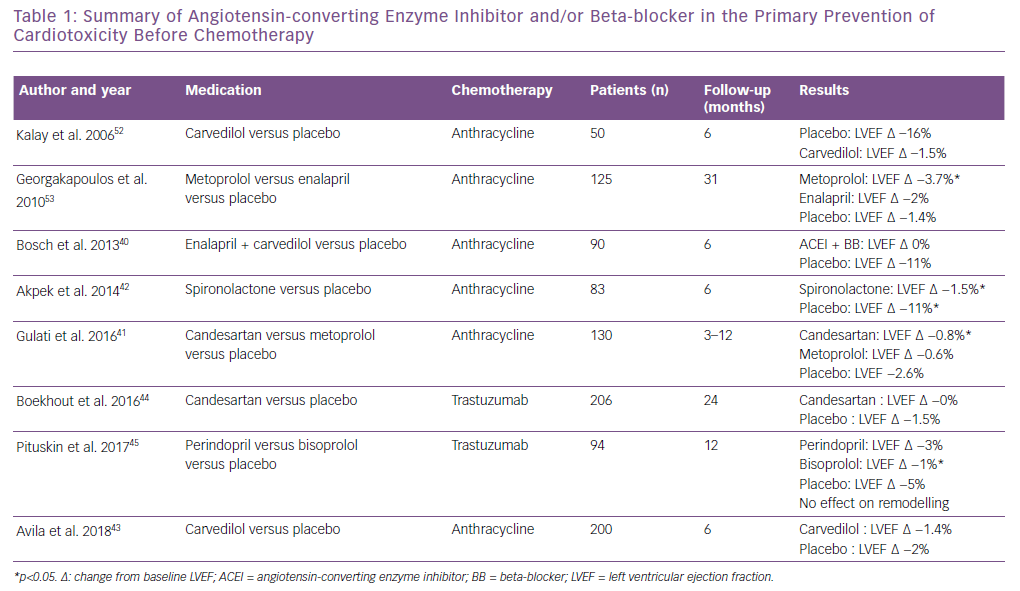
Should We Treat Subclinical LV Dysfunction In Primary Prevention?
There is currently no evidence showing that a cardioprotective treatment, such as angiotensin-converting enzyme (ACE) inhibitor and/or beta-blocker, should be given to all cancer patients undergoing potential cardiotoxic chemotherapy. Table 1 provides a summary of ACE inhibitors and beta-blockers in primary prevention of cardiotoxicity before chemotherapy. Regarding patients treated with anthracycline for haematological malignancies, Bosch et al. showed a preventive effect of enalapril associated with carvedilol.40 The Adjuvant Breast Cancer Therapy (PRADA) trial more recently showed a benefit of angiotensin 2 receptor antagonists (candesartan) to prevent LVEF decrease, but metoprolol had no protective effect.41 Akpek et al. showed a beneficial effect of spironolactone on the prevention of myocardial dysfunction (defined by a 10% decrease of LVEF), but only 80 patients were randomised.42 Recently, the Carvedilol Effect for Prevention of Chemotherapy-Related CardiotoxicitY (CECCY) study, which tested the cardioprotective effect of systemic carvedilol preventive therapy, did not find any benefit in LV dysfunction prevention, but showed a TnI decrease at 6 months compared with a placebo.43 Boekhout et al. did not find any benefit of candesartan in the prevention of trastuzumab-induced cardiotoxicity.44 However, Pituskin et al. showed that perindopril and bisoprolol prevented the LVEF decrease, but had no effect on cardiac remodelling at 1 year compared with a placebo.45
More recently, Cardinale et al. compared the initiation of treatment with enalapril either systematically in prevention or during a troponin increase after receiving an anthracycline-based regimen in a low cardiovascular risk population (4% hypertension, 4% diabetes). The incidence of troponin elevation was 23% in the prevention group (treated with enalapril) and 26% in the non-treated group, respectively; p=0.50. Only three (1.1%) patients developed cardiotoxicity (defined as a LVEF decrease <10 percentage points from baseline to a value <50%), and there was no difference in terms of cardiac dysfunction in the two groups. This study suggests that in a low cardiovascular risk population, systematic treatment does not bring any benefit compared to a strategy guided on cardiac biomarkers increase.46
As reviewed in Table 1, the systematic use of heart failure therapy in the primary setting remains controversial. However, a high cardiotoxicity risk population seems to benefit from an early introduction of heart failure therapy. In contrast, low-risk patients may not benefit from a cardioprotective treatment and be unnecessarily exposed to adverse events, such as hypotension and renal failure.
Should We Treat Subclinical LV Dysfunction If Troponin Increases?
The potential value of a troponin-guided cardioprotective treatment was investigated by Cardinale in 2006 in a prospective randomised study enrolling 473 patients treated with anthracycline. TnI was measured at each chemotherapy administration, and 114 patients showed an increase of TnI. In this population, 1 month after the end of anthracycline treatment, patients were randomised to receive a 1-year enalapril treatment or placebo. At 12 months, no cardiotoxicity (defined as LVEF decrease <10 percentage points from baseline to a value <50%), was noted in the enalapril group, whereas 43% of patients developed a LV dysfunction in the placebo group (p<0.001). Moreover, the incidence of cardiotoxicity was higher in the persistent troponin increase group than those who showed an increase of troponin only during the anthracycline regimen. The placebo group exhibited a higher risk of cardiac events.47
Should We Treat Subclinical LV Dysfunction If Global Longitudinal Strain Decreases?
GLS-guided heart failure therapy is less studied. A small, observational, non-randomised study enrolled 159 patients receiving anthracycline, trastuzumab or both. Fifty-two patients showed a decrease of GLS >–11% at 6 months after baseline evaluation. Of 52 patients, 24 were treated with beta-blockers and 28 with a placebo. After 6 months of treatment, GLS and LVEF significantly improved in the beta-blockers group, but not in the placebo group.48 The Strain SUrveillance During Chemotherapy for Improving Cardiovascular OUtcomes (SUCCOUR) study will give some answers. Indeed, in this study in progress, patients with a relative reduction of GLS by ≥12% are treated with cardioprotective therapy.49
Should We Treat Subclinical LV Dysfunction If LVEF is <53%?
According to the position paper of the Working Group on Cardio-oncology of the European Society of Cardiology, ACE inhibitors and beta-blockers are recommended in patients with asymptomatic cardiac dysfunction to prevent the development of symptomatic heart failure or further dysfunction. This recommendation is based on an observational study, enrolling 2625 patients treated with anthracycline. In the population developing cardiotoxicity (n=226; defined by a decrease of 10 percentage points to a value <50%), ACE inhibitors +/– beta-blockers were initiated early. Among these 226 patients, 82% recovered from cardiotoxicity at least partially with heart failure therapy. Nevertheless, those who failed to improve LVEF had a significantly higher risk of major cardiovascular events.50
The same results have been observed regarding the effect of heart failure therapy in cancer patients receiving anthracycline treatment when LVEF <45% (201 patients).51 Although there was no control group, full LVEF recovery occurred in 42% of patients treated with enalapril and carvedilol. Responders showed a lower rate of cumulative cardiac events than partial and non-responders (5%, 31% and 29%, respectively; p<0.001).
These findings support the fact that early detection of subclinical cardiac dysfunction by LVEF decrease could lead to an early start of heart failure therapy, thus preventing cardiac outcomes.
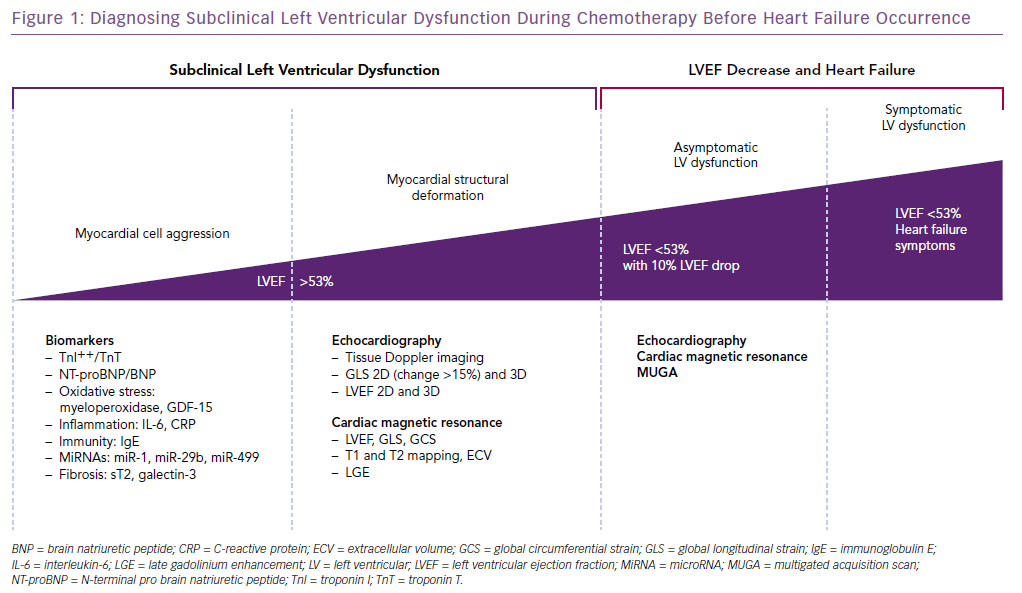
Conclusion
The early detection of myocardial dysfunction during chemotherapy is a major issue. The currently used tools are cardiac biomarkers (especially TnI), global longitudinal strain, and LVEF. According to the above-mentioned studies, systematic preventive treatment with beta-blocker and/or ACE inhibitor has not shown a reduction in cardiac outcomes. However, individualised management of patients with careful evaluation and treatment of cardiovascular risk factors should be performed before the start of chemotherapy. An increase of TnI during chemotherapy should lead to administering cardioprotective ACE antagonist-based treatment. In the case of asymptomatic LVEF decrease, beta-blocker and ACE antagonist should be given. There is no specific management of symptomatic heart failure therapy during chemotherapy. The current definition of cardiotoxicity, which is still based on heart failure symptoms onset or LVEF drop, has to be changed, moving from a clinical to a subclinical definition, based on earlier, more sensitive and specific biomarkers, and new imaging tools based on echocardiography and CMR (Figure 1).







